The Dengue Virus
Dengue is the world’s most prevalent and consequential arboviral disease. Current estimates indicate that as many as 390 million dengue infections occur every year in over 100 countries, and more than 50% of the global population are now at risk of infection (1). The causative agent of dengue infection is the dengue virus (DENV): a positive single-stranded RNA virus of the Flaviviridae virus family, which also includes the Zika virus (ZIKV), as well as yellow fever, and West Nile. DENV is transmitted by the Aedes genus of mosquitoes that circulate in much of the globe’s tropics and subtropics, transmitting the virus as they feed on blood. Aedes circulation was historically constrained to the globe’s hotter, equatorial regions, but recent reports of its presence in Europe and North America are indicative of its expanding distribution (see Figure 1).
Most DENV infections are asymptomatic, but in some cases can cause a wide range of clinical manifestations, from mild fever to the potentially fatal dengue shock syndrome (DSS). As such, the clinical burden of dengue is significant and 95 million people seek medical care every year as a result (2). At least four distinct serotypes of DENV have been identified to date, all of which cause the full spectrum of disease. Infection with one DENV serotype confers long-lasting type-specific immunity. However, secondary infections by other DENV serotypes are frequently associated with more serious forms of the disease (3).
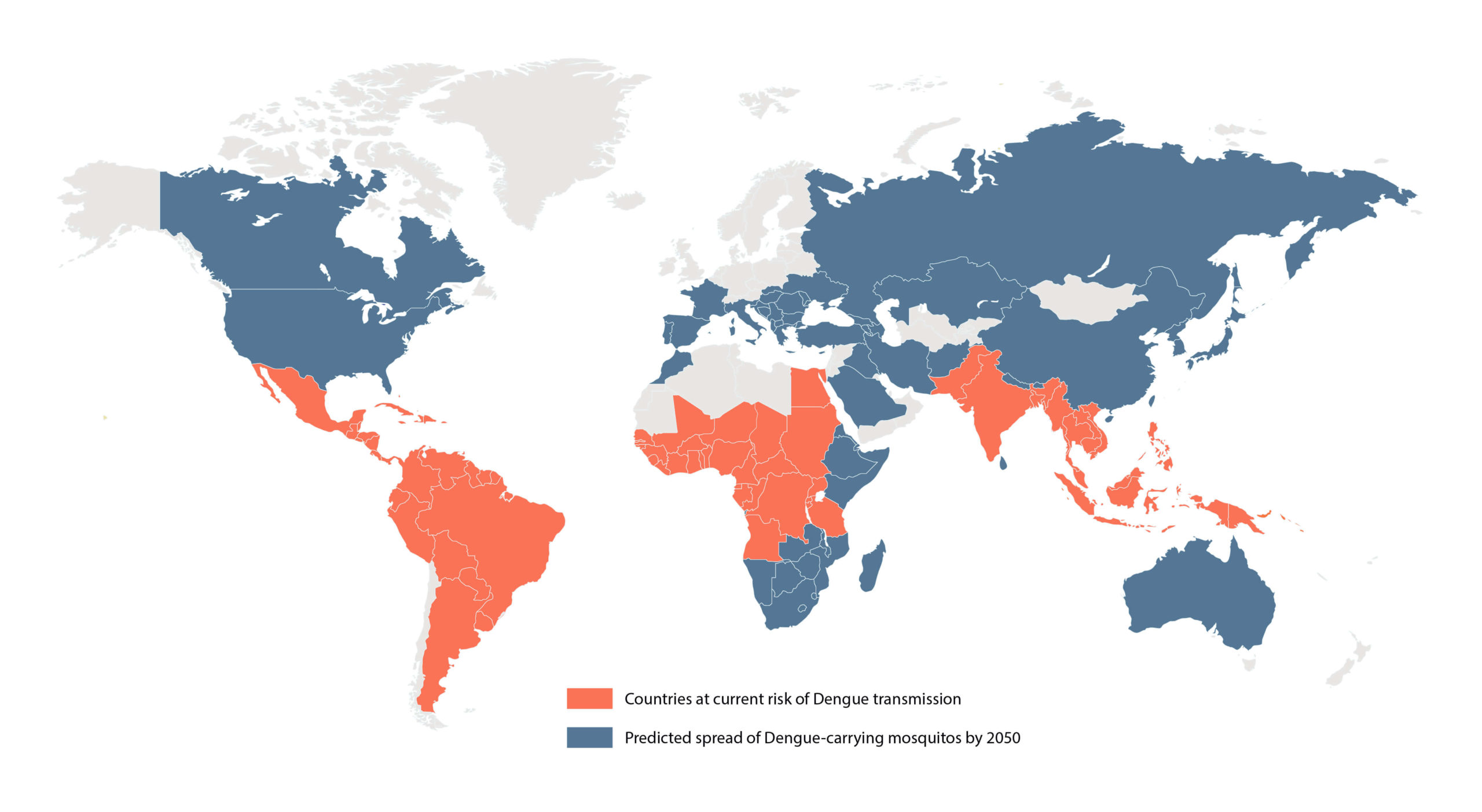
Figure 1: Map of the current and predicted distribution of dengue virus by 2050 (2-3)
Dengue Vaccines
In 2016, Dengvaxia® was the first dengue vaccine to be approved. Dengvaxia (CYD-TDV) is a live attenuated vaccine, comprising structural proteins of the four DENV serotypes that are known to elicit humoral responses during natural DENV infection (6). Following promising data on safety, the vaccine was approved by multiple regulatory agencies and recommended for use in certain populations by WHO. Dengvaxia became available in 11 countries shortly after, and large-scale public vaccination programmes were implemented in Brazil and the Philippines to vaccinate over 1 million children at risk of infection (7).
However, in late 2017, adverse events were reported, which coincided with a retrospective analysis showing that Dengvaxia causes an increased risk of severe infection in individuals who have not had previous DENV infection (i.e., are seronegative) (8). These effects were primarily seen in children and correlated with a lower probability of them having experienced a previous infection, but were likely also influenced by other factors such as the nature of their response to vaccination. The cause of these adverse events is thought to be the result of a phenomenon known as antibody-dependent enhancement (ADE). While the mechanism for ADE is not clear, the prevailing hypothesis is that the immune response to a second infection of a different DENV serotype causes a rapid release of antibodies that bind the ‘new’ virus, but do not effectively neutralise it, and instead facilitate viral entry to host cells (see Figure 2) (9). The consequences of ADE-mediated DENV uptake are a much more severe form of infection that manifests as DSS (10).
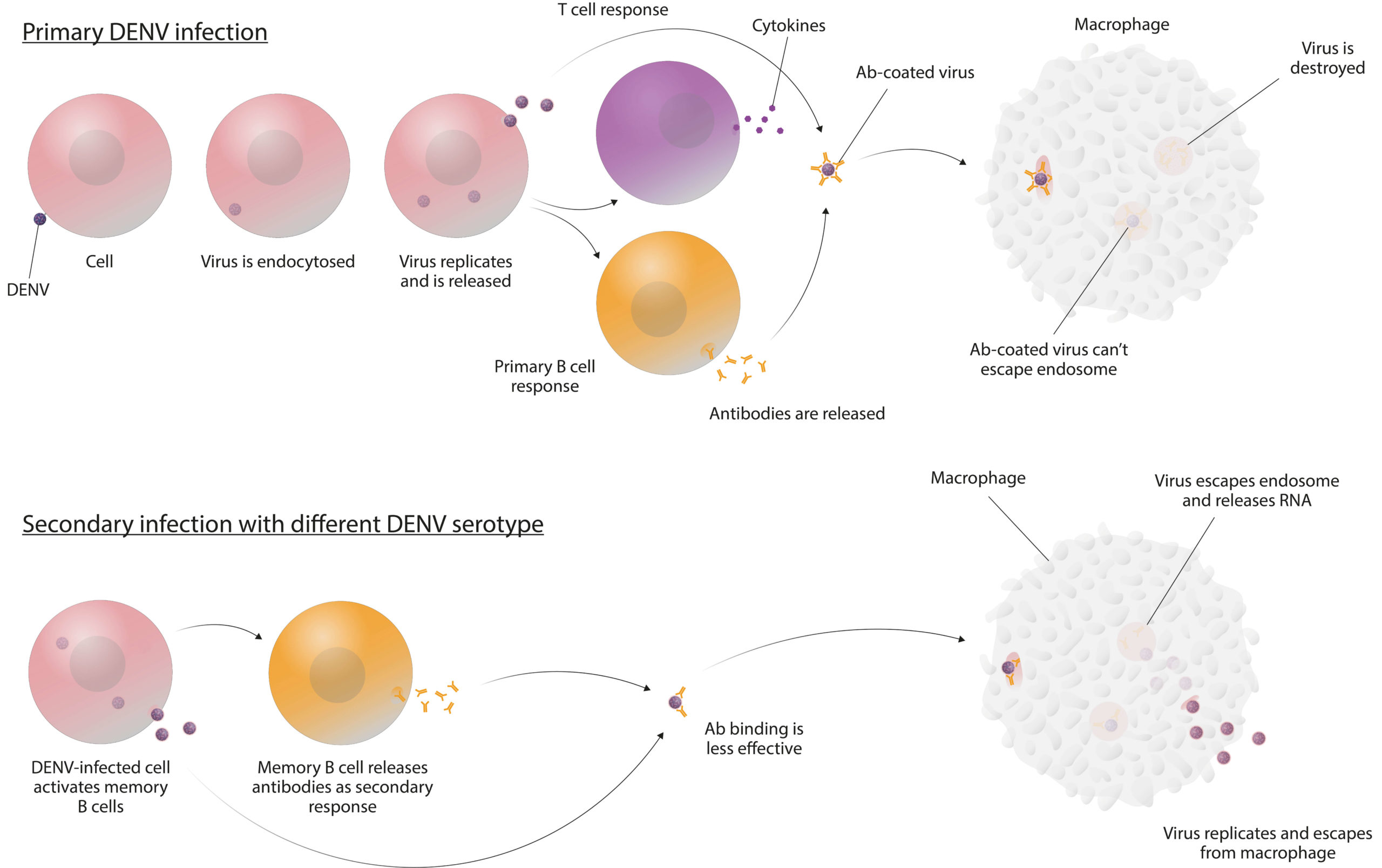
Figure 2: One of the suggested mechanisms of ADE (10)
The possibility that Dengvaxia can cause ADE has led to concerns regarding the safety of mass vaccination programmes. However, in 2018, WHO’s Strategic Advisory Group of Experts concluded that Dengvaxia could play a positive role in public health programmes as long as pre-vaccination serological screening is used to ensure that only individuals who are seropositive receive vaccinations (11). If such vaccination strategies were implemented, millions more could be immunised and, if successful, would result in substantially fewer severe and hospitalised cases of dengue infection. In addition to Dengvaxia, various other dengue vaccine candidates are now in early and late-stage development. Of these, TAK003/005 is a front-runner that has seen success in Phase III clinical trials and may be approaching regulatory approval in the near future. In addition to the structural proteins in Dengvaxia, TAK003/005 includes DENV non-structural protein 1 (NS1). Considering that anti-NS1 antibodies have been shown to confer a protective role and NS1 vaccination can prevent vascular leakage in mice, NS1’s inclusion may confer a greater protective and immunising effect in a vaccine formulation (12). However, while TAK003’s association with ADE is not clear, a recent study has suggested that more work needs to be done to understand the role that anti-NS1 antibodies could play in eliciting ADE responses in vivo (13). Therefore, like Dengvaxia, other vaccine candidates such as TAK003/005 will likely require serostatus testing for post-marketing surveillance and general administration.
Developing Better Diagnostics
Considering the importance of unequivocally identifying DENV serostatus prior to vaccination, the use of accurate diagnostics is crucial. To determine the serostatus of an individual, the presence or absence of anti-DENV antibodies in blood samples should be analysed using a diagnostic test. However, serological diagnostics for the flaviviruses are often impeded by antibody cross-reactivity, whereby antibodies raised to a flavivirus may also bind to another one due to close structural similarities. In particular, DENV’s envelope proteins share a very high degree of structural similarity with those of the ZIKV. As such, DENV and ZIKV are notorious for inducing cross-reactive antibody responses that have plagued the accuracy of serological diagnostics (14). Furthermore, considering that most DENV diagnostics were developed prior to ZIKV’s emergence, there has been insufficient time to develop appropriate alternatives and little is known about their performance in relevant populations (15). Other issues relate to the intended use of DENV diagnostics. For example, as immunoglobulin G (IgG) levels are higher during acute dengue infection, many rapid diagnostic tests (RDTs) for acute diagnosis are not suitable for the detection of lower, post-infection IgG levels in seropositive individuals.
Given the current limitations of existing diagnostics, a new generation of diagnostic tests for pre-DENV vaccine screening are needed that can function reliably across a range of epidemiological settings (16). Most importantly, to mitigate the risk of misdiagnosis, these kits will need to approach 100% specificity and sensitivity.
The Role of Reagents
The key to achieving specificity with any serological diagnostic lies in the identification, selection, and presentation of virus-specific epitopes. Due to safety restrictions, most of the antigens used in studies are expressed in recombinant systems that aim to faithfully represent the structure of the native protein in question. However, the degree to which these biological reagents can vary is significant, and structural fidelity is not always ensured. For example, proteins expressed in more simple systems such as Escherichia coli, lack glycosylation, which alters natural epitopes and may render the antigen inappropriate for serological studies. To ensure reagents are suitably specific for diagnostic assays, expression in mammalian systems is, therefore, preferred to guarantee proper folding and full glycosylation.
A further consideration for the choice of antigens is their specificity in the context of co-circulating ZIKV. Since the Zika epidemic of 2016, it has become clear that the similarity between these flaviviruses makes it very difficult to design diagnostic tests that can reliably differentiate them. While the focus has been on developing more specific ZIKV tests, the specificity of existing dengue tests against the background of co-circulating Zika should be further investigated. To improve reagent specificity, various methods have been explored, such as the screening of antigen arrays alongside mutation studies aimed at removing cross-reactive epitopes.
The choice of antigen should also depend on what a diagnostic assay is trying to achieve. In the context of Dengvaxia where NS1 is not included in the formulation, NS1-based diagnostics can theoretically differentiate between natural infection and vaccination response; for TAK003/005 where NS1 is included in the formulation, being able to measure the response to NS1 and assess the presence of antibodies that may neutralise NS1’s pathogenic effects is crucial. Functional studies that depend on the quality of biological reagents are of particular importance (12).
Both epitope specificity and the abundance of antibody subsets differ between populations and individuals due to genetics, co-circulating pathogens, vaccination status, and history of natural infection. As a result, better performance
and characterisation are needed across populations at risk, and more work is required to convert such tests into rapid point-of-care devices. It also remains a challenge to convert many of the enzyme-linked immunosorbent assays used in early research into functional RDTs. Given the complexity of RDT development, reagent design and formulation are important factors that need to be considered during the early stages of development. With this in mind, there are real advantages for assay developers who work closely with reagent manufacturers to synthesise the precise reagents they need.
References
1. Castro MC et al, Disease and economic burdens of dengue, Lancet Infect Dis 17(3): pp70-8, 2017
2. Kamal M et al, Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate, PLoS One 13(12): pp1-21, 2018
3. Bhatt S et al, The global distribution and burden of dengue, Nature 496(7,446): pp504-7, 2013
4. Tatem AJ et al, Global traffic and disease vector dispersal, Proc Natl Acad Sci USA 103(16): pp6,242-7, 2006
5. Swaminathan S and Khanna N, Dengue vaccine development: Global and Indian scenarios, Int J Infect Dis: pp80-6, 2019
6. Thisyakorn U and Thisyakorn C, Latest developments and future directions in dengue vaccines, Ther Adv Vaccines 2(1): pp3-9, 2014
7. Visit: www.straitstimes.com/world/americas/brazil-launches-first-dengue-vaccine-campaign
8. Visit: www.sanofi.com/en/media-room/press-releases/2017/2017-11-29-17-36-30
9. Visit: www.nature.com/articles/srep29201
10. Visit: www.the-scientist.com/infographics/antibody-dependent-enhanced-ade-immunity-39674
11. Visit: www.who.int/immunization/sage/meetings/2016/april/1_Background_Paper_Dengue_Vaccines_2016_03_17.pdf
12. Sharma M et al, Magnitude and functionality of the NS1-specific antibody response elicited by a live-attenuated tetravalent dengue vaccine candidate, J Infect Dis: pp1-11, 2019
13. Wan SW et al, Therapeutic effects of monoclonal antibody against dengue virus NS1 in a STAT1 knockout mouse model of dengue infection, J Immunol 199(8): pp2,834-44, 2017
14. Chao DY et al, Comprehensive evaluation of differential serodiagnosis between Zika and dengue viral Infections, J Clin Microbiol 57(3): pp1-14, 2019
15. Luo R et al, Rapid diagnostic tests for determining dengue serostatus: A systematic review and key informant interviews, Clin Microbiol Infect 25(6): pp659-66, 2019
16. Javelle E et al, Letter to the editor: False-positive results with rapid diagnostic tests (RDT) for dengue, Euro Surveill 24(21): pp1-2, 2019

