New Norovirus-Like Particles Available
To support norovirus research and development, The Native Antigen Company offers 6 new norovirus-like particles, produced from our proprietary VirtuE™ mammalian expression system.
Our Norovirus-Like Particles
Virus-like particles (VLPs) comprise the structural proteins of a viral particle, but don’t contain genomic material, making them non-infectious, while presenting all of the structural epitopes of a native virus. Because of these properties, VLPs have been extensively used in diagnostic and vaccine R&D over recent years.
To support norovirus research and development, The Native Antigen Company offers an extended range of 8 norovirus-like particles, produced from our proprietary VirtuE™ (HEK293) and insect-baculovirus expression systems.
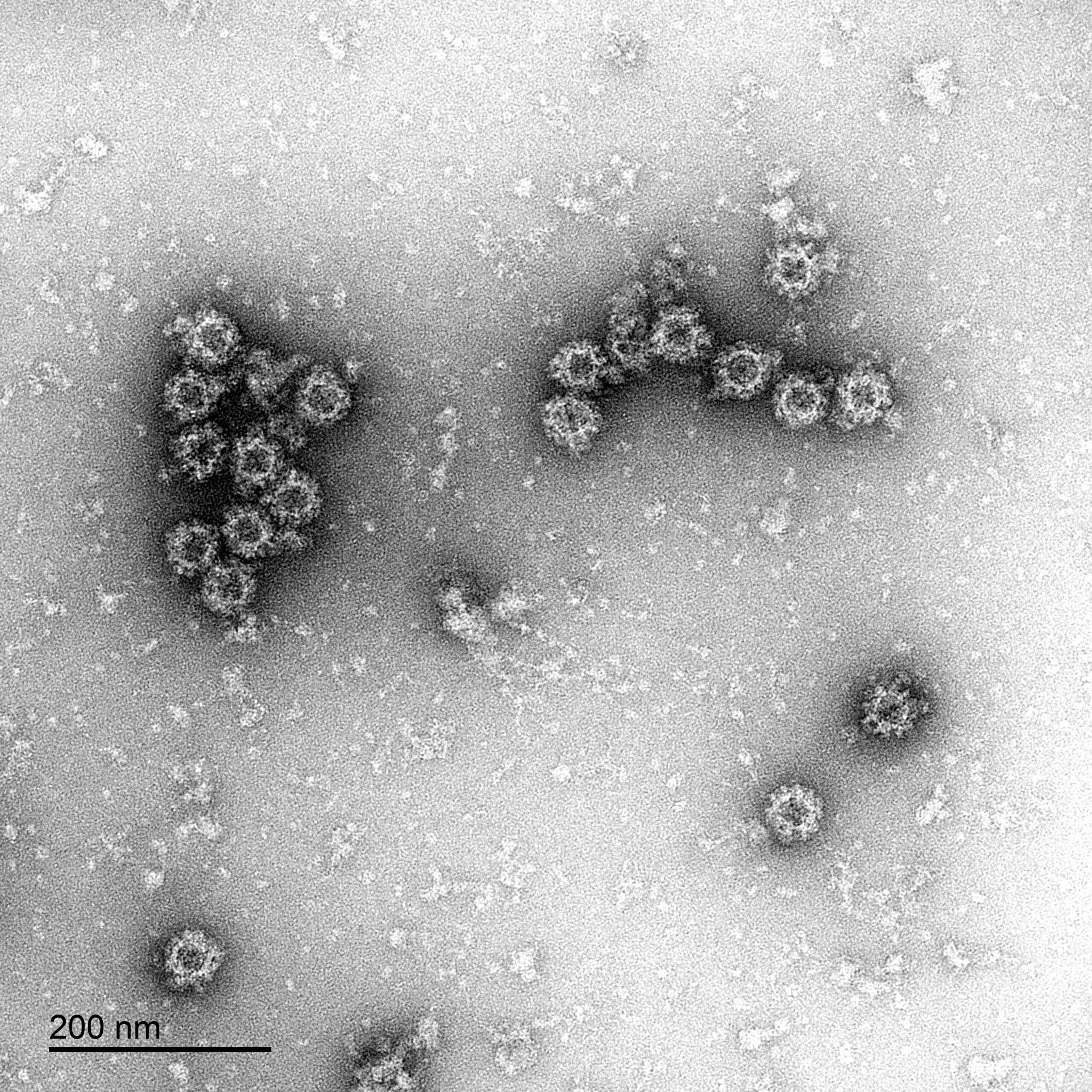
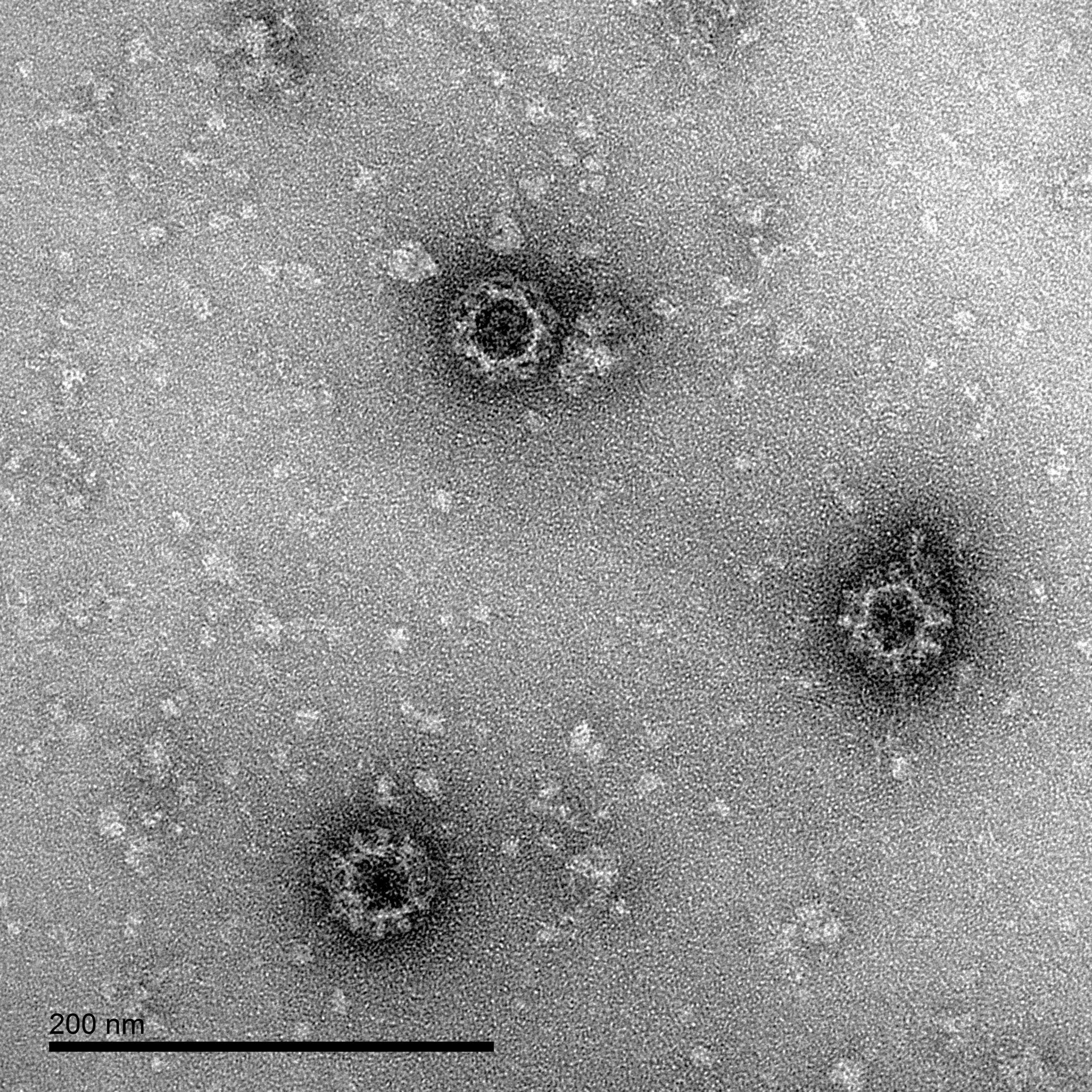
Norovirus Electron Micrographs: negative stain EM of norovirus GI.1 VLPs produced by The Native Antigen Company, showing intact particles.
Our norovirus VLPs are comprised of VP1 proteins, that self-assemble as an icosahedron of 180 subunits. VP1 is a 59kDa glycoprotein with three key domains: The shell domain (S domain) contains elements essential for the formation of the icosahedron. The Protruding domain (P domain) is divided into sub-domains P1 and P2. The P domain interacts in dimeric contacts that increase the stability of the capsid and form the protrusions on the virion. A hypervariable region in P2 is thought to play an important role in receptor binding and immune reactivity.
Our VLPs are non-infectious and safe to use, while displaying native-like epitopes and glycosylation patterns. A study by Metz and colleagues, for example, showed that our VirtuE™-expressed dengue VLPs exhibit comparable epitopes to native viral particles. Our VLPs are suitable for a wide range of applications, including the development of in vitro diagnostic assays and vaccines. The highly repetitive structural patterns presented by VLPs also makes them ideal antigens for raising high-avidity antibodies.
For more information on each of our norovirus-like particles, click the buttons below or get in touch with a member of our team:
See our Full Range
In addition to virus-like particles, we offer a range of genotype-specific primary antibodies:
