In this blog, we speak with Dr. David Flavell about his scientific career, the legacy of Leukaemia Busters, and the recent impact that COVID-19 has had on his research.
Tell me about your scientific background David.
I was born in a seaside town called Southport in the North West of England and in 1971 went to Liverpool John Moores University (Liverpool Polytechnic as it was known then) to do a degree in biological sciences. In 1975, I then moved on to Sheffield University, where I did my PhD in cancer immunology. It was there that I met my wife, Bee, who was also doing a PhD in immunology. We’ve worked together continuously ever since for 44 years!
After gaining our PhD’s we both left for Thailand in 1978 and I set up a laboratory at Siriraj Hospital (Mahidol University) to study the problem of liver fluke infection and a particular type of liver cancer called cholangiocarcinoma common in parts of Thailand. We both moved to The London School of Hygiene and Tropical Medicine (LSHTM) in 1979 to work on the same parasite (Opisthorchis viverrini) to try to figure out how this liver parasite caused cancer. I was then offered a job in the Medical School at Southampton General Hospital in 1983, where I was asked to establish a monoclonal antibody laboratory to produce reagents for lymphoma diagnosis in the department of Professor Dennis Wright – a well-respected pathologist in lymphoma diagnosis and classification who did some of the original work on Burkitt lymphoma and who sadly passed away just a few days ago from a hospital acquired (nosocomial) COVID-19 infection.
How did you come to set up Leukaemia Busters?
In 1988, our son Simon Flavell was diagnosed with T-cell acute lymphoblastic leukaemia. Given that our laboratory was directly involved in producing diagnostic antibodies for haematological malignancies and that Simon was a patient at Southampton General, it was obvious to us that we had to pivot our work towards developing therapeutic antibodies. In 1989, a year before Simon died at the age of ten, we set-up Leukaemia Busters that he gave the name and logo to – a charity devoted to the development of antibody-based treatments for leukaemia. Determined to make a difference after we lost Simon, we developed a number of immunotoxins for use in lymphoma and leukaemia patients and carried out the first early phase clinical trial in Britain of an antibody-based drug called BU12-SAP in children with pre-B cell acute lymphoblastic leukaemia (ALL) between 2001 and 2002 using an anti-CD19 monoclonal antibody coupled to the toxin saporin.
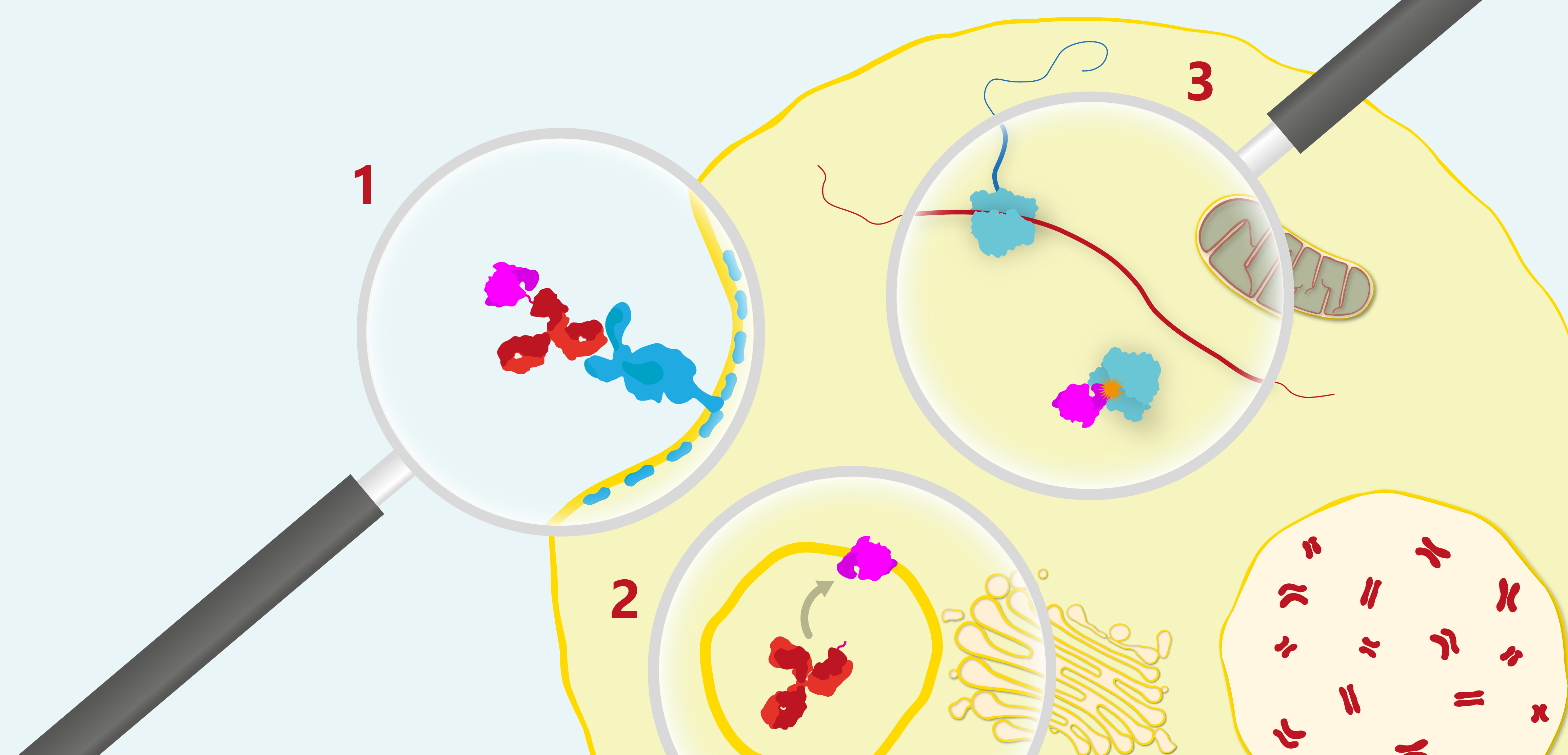
General mechanism of the therapeutic action of a ribosome-inactivating immunotoxin. 1) The antibody binds the cell-surface receptor of a target cell and is endocytosed via a clathrin-dependent pathway, 2) The toxin is proteolytically cleaved from the antibody by an endosomal reductase and escapes the endosome into the cytosol where target ribosomes reside (note that it is not yet clear whether the saporin component of BUP12-SAP is cleaved within the endosome or whether it escapes into the cytosol as an intact conjugate), 3) Once in the cytosol the toxin catalytically depurinates the 28S ribosomal subunit to irreversibly halt protein synthesis bringing about apoptotic cell death.
Unfortunately, the BU12-SAP study came to halt partially because of new EU regulations that made manufacturing costs prohibitively expensive. Inclusion criteria were also very stringent for young children that were sick and in poor health to start with and this did make the study difficult, both practically and emotionally. But the main reason for the premature closure of the trial was the EU clinical trial Directive in 2003 that derailed the whole effort because the new regulations made the full GMP manufacture of BU12-SAP prohibitively expensive. It had been going so well, and we were very disappointed that the study was halted due to events and new regulations that were beyond on our control. We named our new laboratory that officially opened in 1993 The Simon Flavell leukaemia Research Laboratory in his memory, to continue developing therapeutics for leukaemia. It’s an active living memorial to Simon’s memory, and we continue to carry out research there that we hope will be of benefit to other patients like him everywhere.
We had also developed a further three candidate immunotoxins with anti-CD38, -CD7 and -CD22 specificities, but we were only able to get one of these into Phase I trials: OKT10-SAP, an anti-CD38 immunoconjugate for multiple myeloma patients. Instrumental in getting the paediatric trial underway was one of my old MD students from 1990, Bruce Morgan, who was also the paediatric oncology senior registrar while Simon was being treated for his leukaemia at Southampton General Hospital. By this point in time, Bruce had become the lead for new therapeutic agents as part of the United Kingdom Cancer Study Group, and his enthusiasm and belief in the potential value of BU12-SAP from the MD he gained while working with caused him to back the idea of a clinical trial.
That’s an amazing story David and a fantastic cause. What have you been working on since then?
Since then, we’ve continued to work on antibody-based therapeutics – specifically to improve the therapeutic index of our candidates. One of the things we’ve been looking at in particular, is to devise a way of improving the uptake and release of these drugs from endosomes with saponins.
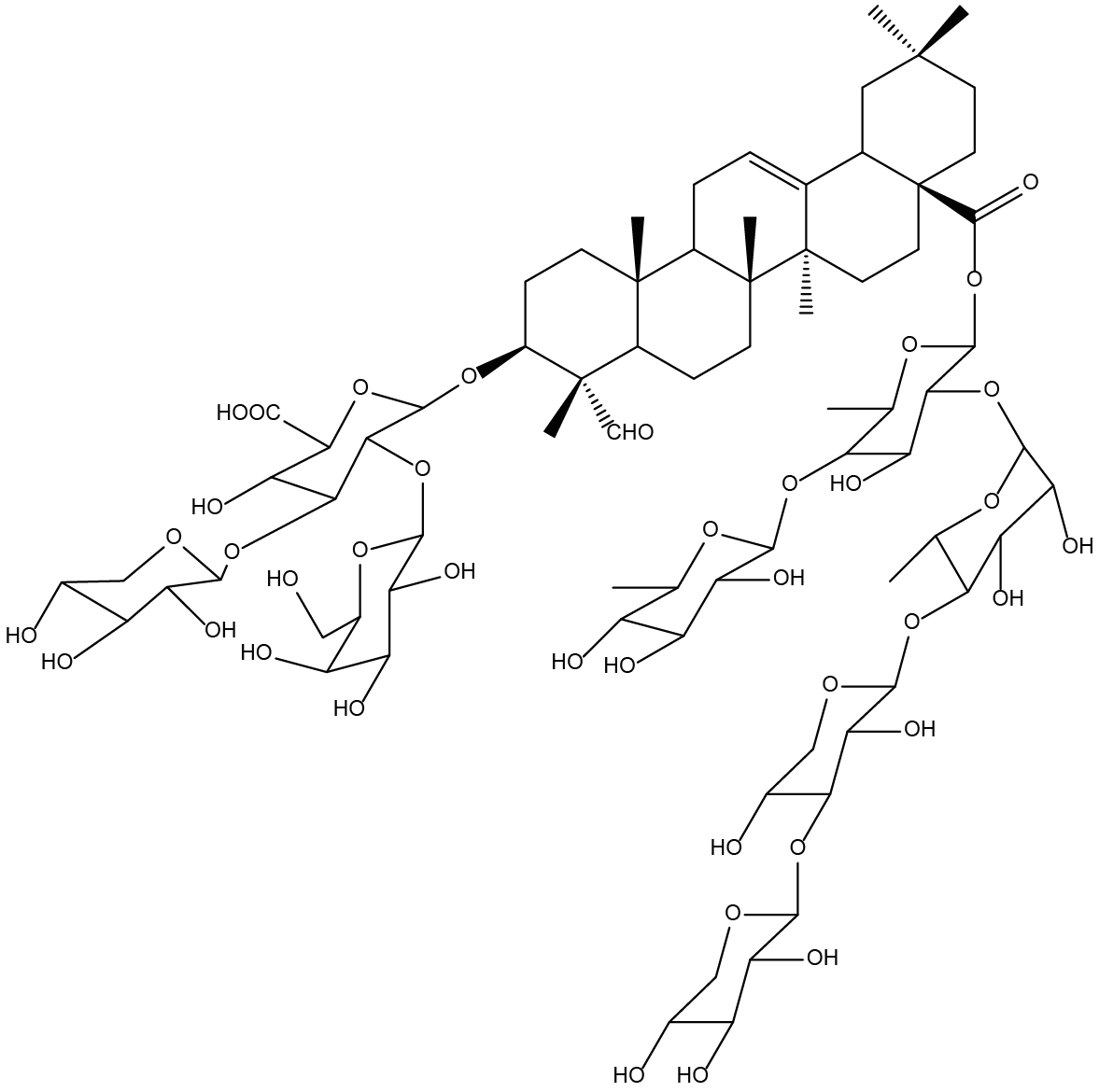
Chemical structure of the saponin from Gypsophila panniculata (Baby’s Breath). Saponins are naturally-occurring, detergent-like molecules, based on a triterpenoid skeleton with two oligosaccharide chains.
Saponins are able to destabilise cell membranes by interacting with cholesterol, which we and others have found very significantly augment the activity of immunotoxins by many thousand-fold. The caveat, however, is that while they are capable of increasing immunotoxin cytotoxicity, this is accompanied by a reduction in immunospecificity. However, this can be largely overcome by scheduling one before the other to restore the immunospecificity. Ultimately the idea would be to formulate the components in a lipid nanoparticle (LNP) or as a conjugate with some form of carrier molecule.
And so what have you been working on most recently David?
We recently developed a novel assay to detect endosomal escape following internalisation of the immunoconjugate into the cell. We’d originally be using confocal microscopy, but could only see escape into the cytosol at higher-than-optimal concentrations of saponin. So, my PhD student and I thought maybe we could repurpose another assay. And so he developed a flow cytometric technique that uses pulse width analysis. It’s a very sensitive technique that detects endosomal escape and it correlates very well with the confocal data for the higher concentrations.
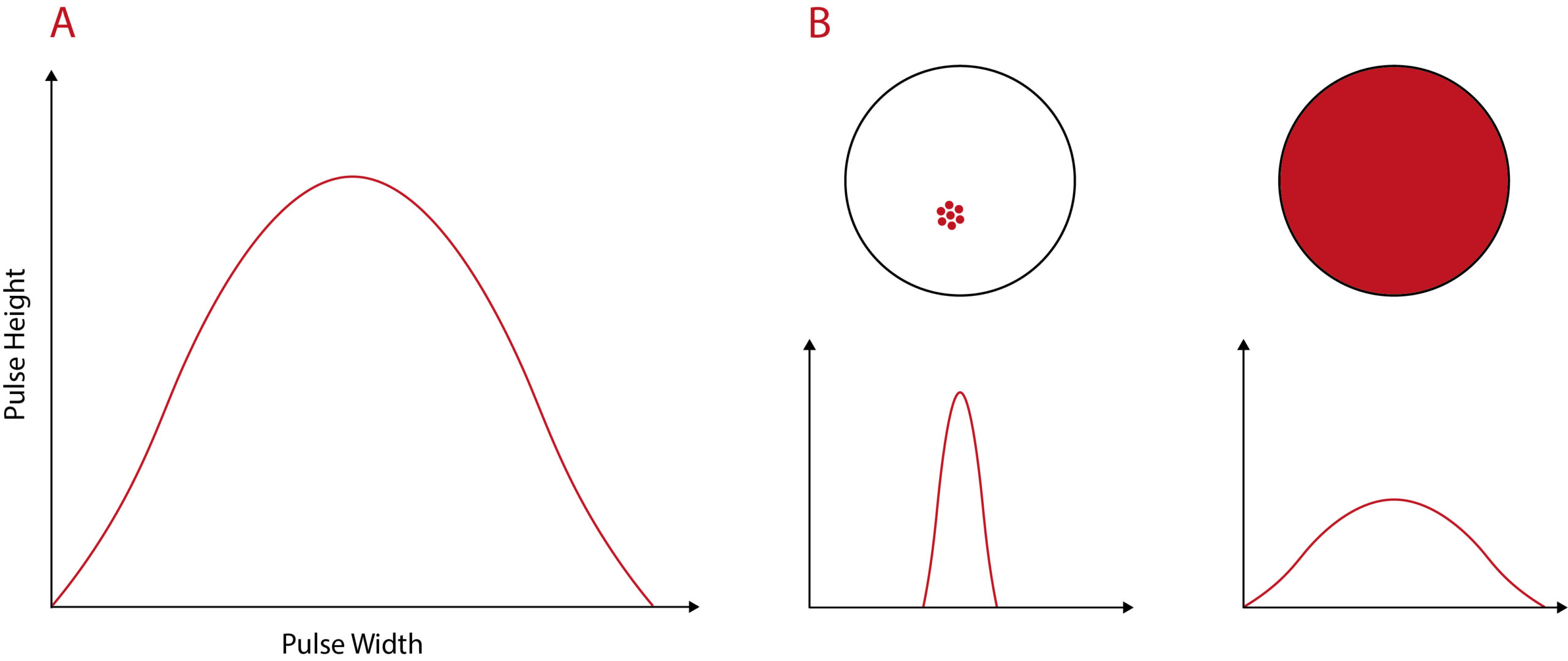
Diagram to illustrate pulse width analysis. A) Flow cytometry signal pulse for a fluorescent particle. The pulse height is the maximum fluorescence intensity. Pulse width represents the amount of time taken for the particle to pass through the laser. (B) The distribution of a fluorescent label within the cell changes the fluorescent pulse width. A localised, vesicular distribution will result in a narrow pulse width. In contrast, fluorescent label with a diffuse, cytosolic distribution will be recorded as a wide pulse width. Diagram adapted from Wensley et al.
The COVID-19 pandemic has put a lot of scientific research on hold. How has it affected your work?
Understandably, our laboratory was shut down following the government lockdown, causing our research to grind to a halt. The NHS may requisition some of our equipment and space for diagnostic use but we’re not yet sure. Fortunately, my PhD student managed to finish his work just before the lockdown and was examined by an academic who flew in from Italy just a few days before the lock down in February.
So we decided, what can we do? From the beginning of the pandemic, it was very clear to us that some aspects of our research crossed over with COVID-19. It has also become quite apparent that there have been some big mistakes made in the UK since the beginning of the pandemic, and we continued to keep our finger on the pulse with other people we know in the field. And so I began the COVID-19 blog for the those without any particular scientific background – the ‘man in the street’ as it were.
For me, it’s become a really important daily exercise, to try to blog something out regularly, especially since we may be easing lockdown soon. We think it’s very important that the UK government doesn’t blunder into the same policy mistakes that were made in the recent past and if our little blogs highlighting these areas can have an influence and help prevent this from happening, then I for one will be happy in circumstances that are far from happy for anyone at the moment.