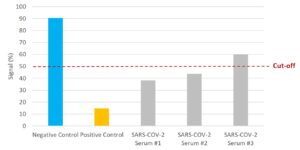
NTRL Assay: Binding rate (B/B0%) for negative samples is ≥ 60%. Binding rate for strongly positive samples is ≤ 50%. Borderline samples, with moderate neutralization, may be assessed with binding rates between 50-60. Negative and positive control antibodies were included. SARS-CoV-2 serum samples #1 and #2 are <50% and therefore positive for the presence of neutralizing antibodies. Serum sample #3 is 60%, indicating no neutralizing capacity.
SARS-CoV-2 Neutralization Assay Development Kit (Kerala Variant RBD-ACE2)
$3,716.26 excl. VAT
KERALA (KAPPA/B.1.617.1) VARIANT
This SARS-CoV-2 Neutralization Assay Development Kit (Kerala variant RBD-ACE2) is intended for the identification and qualitative measurement of neutralizing antibodies. Kit contains enough reagents for 10 x 96-well plates. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
SARS-COV-2 NEUTRALIZATION ASSAY DEVELOPMENT KIT (KERALA VARIANT RBD-ACE2)
This SARS-CoV-2 Kerala Variant Neutralization Assay Development Kit (RBD-ACE2) is intended for the identification and qualitative measurement of neutralizing antibodies. The qualitative immunoenzymatic determination of neutralizing antibodies is based on the ELISA (Enzyme-linked Immunosorbent Assay) technique. Microplates are coated with RBD to bind corresponding ACE2 or blocking antibodies of the sample. After washing the wells to remove all unbound sample material, sample containing the antibody of interest is added and allowed to bind. After incubation a horseradish peroxidase (HRP) labelled ACE2 conjugate is added and incubated. This conjugate binds to the captured RBD which has not been bound by the antibody sample. In a second washing step unbound conjugate is removed. ACE2-HRP conjugate which has bound (and therefore represents the absence of neutralizing antibody), is visualized by adding Tetramethylbenzidine (TMB) substrate which gives a blue reaction product. Sulphuric acid is added to stop the reaction. This produces a yellow endpoint colour. Absorbance at 450nm is then read using a suitable microwell plate reader.
PRODUCT DETAILS – SARS-COV-2 NEUTRALIZATION ASSAY DEVELOPMENT KIT (KERALA VARIANT RBD-ACE2)
- SARS-CoV-2 Kerala Variant Neutralization Assay Development Kit (RBD-ACE2).
- Designed for the qualitative assessment of blocking antibodies to the SARS-CoV-2 Indian (Kerala) variant (B.1.617.1) RBD.
- Kit contains 4 reagents; positive and negative control antibodies, B.1.617.1 variant RBD protein and HRP labeled ACE2.
- This kit contains reagents for 10 x 96-well plates.
- Buffers and plasticware are not included.
BACKGROUND
SARS-CoV-2 is a respiratory virus which causes coronavirus disease 2019 (COVID-19). It spreads primarily through contact with an infected person via respiratory droplets generated when a person coughs or sneezes, or through droplets of saliva or discharge from the nose. The incubation period is believed to range from 2-11 days. Infection with SARS-CoV-2 can cause mild symptoms including a runny nose, sore throat, cough, and fever. Indeed, the majority of COVID 19 cases (about 80%) are asymptomatic or show mild symptoms. However, it can be more severe for some people and may lead to pneumonia or breathing difficulties. The elderly, and people with pre-existing medical conditions (such as, diabetes and heart disease) are more vulnerable to becoming severely ill with the virus.
The SARS-CoV-2 genome encodes four major structural proteins, which are the spike (S) protein, nucleocapsid (N) protein, membrane (M) protein and the envelope (E) protein, each of which is essential to produce a viral particle. The SARS-CoV-2 spike (S) glycoprotein is a class I viral fusion protein on the outer envelope of the virion that plays a critical role in viral infection by recognizing host cell receptors and mediating fusion of the viral and cellular membranes. The S glycoprotein is synthesized as a precursor protein which is then cleaved into an amino (N)-terminal S1 subunit and a carboxyl (C)-terminal S2 subunit. Three S1/S2 heterodimers assemble to form a trimer spike protruding from the viral envelope. The S1 subunit contains a receptor-binding domain (RBD), while the S2 subunit contains a hydrophobic fusion peptide and two heptad repeat regions that mediates the fusion of the viral and host cell membranes. SARS-CoV-2 S protein initially binds to the ACE2 receptor on the host cell via the S1 receptor-binding domain. The S1 domain is then shed from the viral surface, allowing the S2 domain to fuse to the host cell membrane. This process is dependent upon activation of the S protein, by cleavage at two sites (S1/S2 and S2’) via the proteases Furin and TMPRSS2. Furin cleavage at the S1/S2 site may lead to conformational changes in the viral S protein that exposes the RBD and/or the S2 domain. TMPRSS2 cleavage of the SARS-CoV-2 S protein is believed to enable the fusion of the viral capsid with the host cell to allow viral entry. This pathway makes these proteins key targets for drug development and viral inhibition.
BIBLIOGRAPHY
- Hu, B., Guo, H., Zhou, P. et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19, 141–154 (2021).
- World Health Organization (WHO), 2021.
Judith Wellens et al. Combination therapy of infliximab and thiopurines, but not monotherapy with infliximab or vedolizumab, is associated with attenuated IgA and neutralisation responses to SARS-CoV-2 in inflammatory bowel disease. medRxiv 2021.10.13.21264916