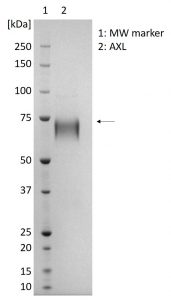
SDS-PAGE showing 1µg purified AXL protein (indicated by arrow).
Human AXL, His-tag
Price range: $763.05 through $2,690.62 excl. VAT
Recombinant AXL protein produced in HEK293 cells and purified from culture supernatant. Protein contains a C-terminal 6x His-tag.
TYROSINE-PROTEIN KINASE RECEPTOR UFO (AXL) – RECOMBINANT PROTEIN
Recombinant AXL protein produced in HEK293 cells and purified from culture supernatant. Protein contains a C-terminal 6x His-tag.
PRODUCT DETAILS – TYROSINE-PROTEIN KINASE RECEPTOR UFO (AXL) – RECOMBINANT PROTEIN
- Recombinant AXL protein produced from HEK293 cells (NCBI Accession Number: NP_068713.2).
- Includes amino acids 1-445 and a C-terminal His-tag.
- Greater than 95% purity by SDS-PAGE and buffered in DPBS, pH7.4.
BACKGROUND
Human tyrosine-protein kinase receptor UFO (AXL) is a cell surface receptor enzyme encoded by the AXL gene (O’Bryan, et al., 1991; Wu, et al., 2014). The AXL protein is characterized by an extracellular structure consisting of two fibronectin type 3-like repeats and two immunoglobulin-like repeats along with its intracellular tyrosine kinase domain. It transduces signals from the extracellular matrix into the cytoplasm by binding to the vitamin K-dependent protein growth arrest-specific 6 (Gas6). It is also is a key facilitator of immune escape and drug-resistance by cancer cells, leading to aggressive and metastatic cancers (Davidsen, et al., 2017).
Viruses use several strategies to facilitate efficient binding to target cells including receptor mediated binding, where a single virus envelope protein targets multiple host cell receptors or multiple envelope proteins which target a single host cell receptor. Viruses can also utilise soluble proteins present in bodily fluids to bridge the virus to target cells. AXL agonist growth arrest-specific 6 (GAS6) protein enhances infectivity of viral envelope proteins by mediating binding of the virus to its target cells by bridging virion envelope phosphatidylserine to AXL (Bhattacharyya, et al., 2013; Schmid, et al., 2016; Morizono, et al., 2011).
AXL has been shown to enhance both pseudotyped and live Ebola virus infection. Ebola virus has a broad cell tropism involving both calcium-dependent lectins and β1 integrin. It also utilises several receptor-type tyrosine kinases, including AXL. Ectopic expression of these molecules in lymphocytes, which normally are highly resistant to infection, enhances Ebola virus GP-mediated infection (Shimojima, et al., 2007; Shimojima, et al., 2006; Wang, et al., 2017).
Ablation of AXL in mice has been demonstrated to enhance susceptibility to infection by Influenza A virus and West Nile virus. Additionally, fewer of the T cells matured to the stage where they could attack the virus in the mice. AXL is also a Dengue virus entry factor. Cells poorly susceptible to these viruses are robustly infected after ectopic expression of AXL receptors. Conversely, virus infection of susceptible cells is inhibited by antibodies or knockdown of AXL expression (Meertens, et al., 2012). AXL has also been shown to contribute to Lassa Fever virus entry in the absence of its major cellular receptor, extracellular matrix receptor dystroglycan (Fedeli, et al., 2018).
AXL is believed to be an entry receptor for Zika virus and does serve as an attachment factor for virus on the cell surface of human fibroblast cells (Persaud, et al., 2018; Meertens, et al., 2012).However, it has also been demonstrated that AXL knockout does not block the entry of Zika virus into primary human astrocytes and astrocytoma cell lines. The presence of AXL instead promotes infection in human astrocytes by antagonizing type I IFN signalling (Chen, et al., 2018).
AXL is therefore a useful target for studying nonspecific virus infectivity and has been proposed as a potential target for future antiviral therapies (Sarukhanyan, et al., 2018).
References
- Bhattacharyya, S., Zagórska, A., Lew, E. D. & et al., 2013. Enveloped Viruses Disable Innate Immune Responses iDendritic Cells by Direct Activation of TAM Receptors Cell Host Microbe. Cell Host Microbe, 14(2), p. 136–147.
- Chen, J., Yang, Y. & et al., 2018. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nature Microbiology, Volume 3, p. 302–309.
- Davidsen, K. T., Haaland, G. S., Lie, M. K. & Lorens, J. B., 2017. The Role of Axl Receptor Tyrosine Kinase in Tumor Cell Plasticity and Therapy Resistance. In: L. Akslen & R. Watnick, eds. Biomarkers of the Tumor Microenvironment. s.l.:Springer, pp. 351-376.
- Fedeli, C., Torriani, G. & et al., 2018. Axl Can Serve as Entry Factor for Lassa Virus Depending on the Functional Glycosylation of Dystroglycan. J Virol., 92(5).
- Meertens, L., Carnec, X. & et al., 2012. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe, 12(4), p. 544–557.
- Morizono, K., Xie, Y. & et al., 2011. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe, 9(4), p. 286–298.
- O’Bryan, J. P. et al., 1991. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Molecular and Cellular Biology, 11(10), p. 5016–31.
- Persaud, M., Martinez-Lopez, A. & et al., 2018. Infection by Zika viruses requires the transmembrane protein AXL, endocytosis and low pH. Virology, 18(3), pp. 301-312.
- Sarukhanyan, E., Shityakov, S. & Dandekar, T., 2018. In Silico Designed Axl Receptor Blocking Drug Candidates Against Zika Virus Infection. ACS Omega, 3(5), pp. 5281-5290.
- Schmid, E. T., Pang, I. K. & et al., 2016. AXL receptor tyrosine kinase is required for T cell priming and antiviral immunity. eLife, Volume 5.
- Shimojima, M., Ikeda, Y. & Kawaoka, Y., 2007. The Mechanism of Axl-Mediated Ebola Virus Infection Masayuki Shimojima Yasuhiro Ikeda Yoshihiro Kawaoka. The Journal of Infectious Diseases, 196(2), p. S259–S263.
- Shimojima, M., Takada, A., Ebihara, H. & et al., 2006. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol., Volume 80, pp. 10109-16.
- Wang, Z. Y., Wang, Z., Zhen, Z. D. & et al., 2017. Axl is not an indispensable factor for Zika virus infection in mice. J Gen Virol., 98(8), pp. 2061-2068.
- Wu, X. et al., 2014. AXL kinase as a novel target for cancer therapy. Oncotarget, 5(20), p. 9546–63.