Image by Virology Research Services Ltd
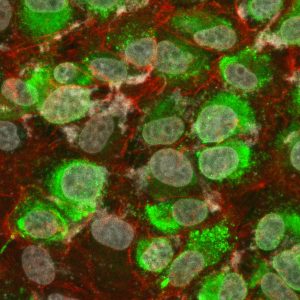
Immunofluorescence: Immunofluorescent staining of Dengue virus serotype 2 infected cells using MAB12135.
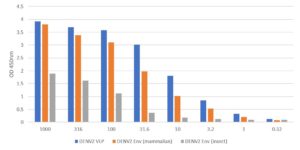
ELISA: Antigen-down ELISA using MAB12135 to detect recombinant Dengue virus serotype 2 envelope protein produced in mammalian HEK293 cells (DENV2-ENV) and in baculovirus infected insect cells (REC31680).
MOUSE ANTI-DENGUE VIRUS VLP/ENV PROTEIN SEROTYPE 2 ANTIBODY (ME1)
This Mouse anti Dengue virus serotype 2 monoclonal antibody is specific for Dengue virus serotype 2. The antibody binds both to native Dengue virus serotype 2, and to recombinant Dengue virus serotype 2 virus-like particles (from transiently transfected HEK293 cells) and envelope protein (from HEK293 cells and baculovirus-infected insect cells). The antibody binds to a conformational epitope on the virus particle, and is not reactive in Western Blotting. Antibody does not recognise DENV envelope protein domain III (EDIII). This Mouse anti Dengue virus serotype 2 antibody is suitable for use in direct ELISA and in Immunofluorescence applications. The antibody is not suitable for Western blot.
PRODUCT DETAILS – MOUSE ANTI-DENGUE VIRUS VLP/ENV PROTEIN SEROTYPE 2 ANTIBODY (ME1)
- Mouse anti-Dengue virus VLP/Envelope monoclonal IgG1 Kappa antibody (clone ME1).
- Greater than 95% purity by SDS-PAGE and buffered in PBS, pH 7.4.
BACKGROUND
Dengue is an emerging disease and a leading cause of illness and death in the tropics and subtropics, with more than one-third of the world’s population living in areas at risk of infection. As many as 100 million people are infected yearly. Dengue is caused by any one of four related viruses transmitted by mosquitoes. Dengue virus (DENV) is a single-stranded RNA virus that belongs to the genus Flavivirus, which includes the Zika virus, West Nile virus and Japanese Encephalitis virus. Currently, there is no antiviral therapy for the treatment of dengue or severe dengue disease. Prophylactic vaccines are in development and the first dengue vaccine, Dengvaxia (CYD-TDV) a live recombinant tetravalent vaccine by Sanofi Pasteur, was licensed in Mexico in December 2015, with several other vaccine candidates in clinical or pre-clinical stages of development.
Early diagnosis of dengue and dengue serotypes is a vital step in patient management. However, many of the proteins expressed by Flaviviruses are very similar and serological testing is complicated by problems of cross-reactivity. In this context, the use of highly purified antigens, that are glycosylated and natively folded are crucial in the development of accurate and reliable immunoassays.
REFERENCES