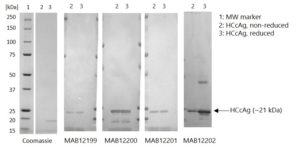
Western Blot: 100ng of Hepatitis C virus recombinant core protein (HCcAg) was separated by SDS-PAGE, under both reducing and non-reducing conditions. Proteins were transferred using Transblot for 7 min. at 25V. 5% dry milk in PBS-T was used as blocking buffer and dilution buffer for antibodies. Primary antibodies were diluted 1:1,000 and goat anti-mouse-IgG-HRP secondary antibody (Biorad 103005) was used at 1:1,000. The primary antibodies were incubated overnight at 2-8°c and all other steps were carried out for 1h at room temperature with gentle rocking. KPL Membrane TMB was used for detection. Development time approximately 30 sec.
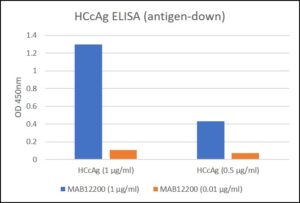
ELISA: Hepatitis C Virus Core Antigen (HCcAg) coated at 0.5µg/ml and 1µg/ml at RT in DPBS for an hour. Plate washed 1 X 300µl/well TBS + 0.1% Tween20, blocked 300µl/well DPBS+1% BSA 1h at room temperature. Detection antibodies diluted to 1.0µg/ml and 0.01µgml in DPBS + 1% BSA + 0.05% T20. Added at 100ul/well, incubated shaken 2h room temperature. Plate washed 3 X 300µl/well TBS-T wash buffer. Biorad goat anti-mouse IgG-HRP secondary antibody (103005) diluted 1 in 10000 in DPBS/1%BSA/0.05%T20, added at 100µl/well, incubated shaken 1h room temperature. Plate washed 6X 300µl/well TBS-T wash buffer. Detection using KPL/Seracare SureBlue TMB substrate added at 100µl/well and the plate developed for 5 min. static on the bench. Reaction stopped with 100µl/well 1M HCL and the plate was read within 3.5 min. at 450nm.
MOUSE ANTI-HEPATITIS C VIRUS CORE PROTEIN ANTIBODY (1863)
Mouse anti HCV core protein antibody is specific for the core protein of Hepatitis C virus (HCV). Mouse anti HCV core protein antibody recognises HCV genotypes 1a and 2. The antibody is suitable for immunoassay research and development.
PRODUCT DETAILS – MOUSE ANTI-HEPATITIS C VIRUS CORE PROTEIN ANTIBODY (1863)
- Mouse anti-Hepatitis C virus core protein monoclonal IgG1 antibody (clone 1863).
- Greater than 90% purity by SDS-PAGE and buffered in PBS, pH7.2.
BACKGROUND
Hepatitis C virus (HCV) is an enveloped, positive-sense, single stranded RNA virus that is a member of the hepacivirus genus of the family Flaviviridae. HCV was first recognised in 1970 and described as non-A, non-B hepatitis, until 1989 when the pathogen was identified as hepatitis C. Currently, eleven genotypes of HCV are recognised, designated 1-11. Genotypes 1-6 are the major genotypes, which are further classified into subtypes a,b and c. Genotype 1 is the most prevalent globally, followed by 3, 2 and 4.
Humans are the primary reservoir of Hepatitis C virus. HCV is a bloodborne virus that is transmitted through infected blood. Transmission of HCV may occur through the sharing of needles for injecting drugs, the use of inadequately sterilised medical equipment infected with HCV and the transfusion of unscreened blood and blood products. HCV is responsible for 15-20% of cases of acute hepatitis worldwide (WHO).
All recognised genotypes are pathogenic and target hepatocytes. The incubation period for HCV infection is 2-6 months. During the period of acute infection, most individuals remain asymptomatic and recover without need for treatment but may present with liver damage later in life. A large percentage of HCV infected Individuals have clinical symptoms that include nausea, vomiting, fatigue, abdominal pain and jaundice. Patients also develop chronic hepatitis, which can progress to cirrhosis or hepatocellular carcinoma. Additional complications associated with persistent HCV infection include insulin resistance and type II diabetes mellitus (Li, HC).
The genotypes of HCV respond differently to treatment and therefore a correct diagnosis is important. However, Hepatitis C virus is difficult to isolate, and the asymptomatic nature of HCV infection makes clinical diagnosis difficult. Serological methods to detect HCV IgM antibodies in patient’s serum are reported to be unreliable but diagnostic methods to detect HCV total antibody and HCV core protein may have some value.
REFERENCES
</ul