Human anti CMV glycoprotein B IgM (P1280), recombinant
Price range: $519.02 through $2,205.79 excl. VAT
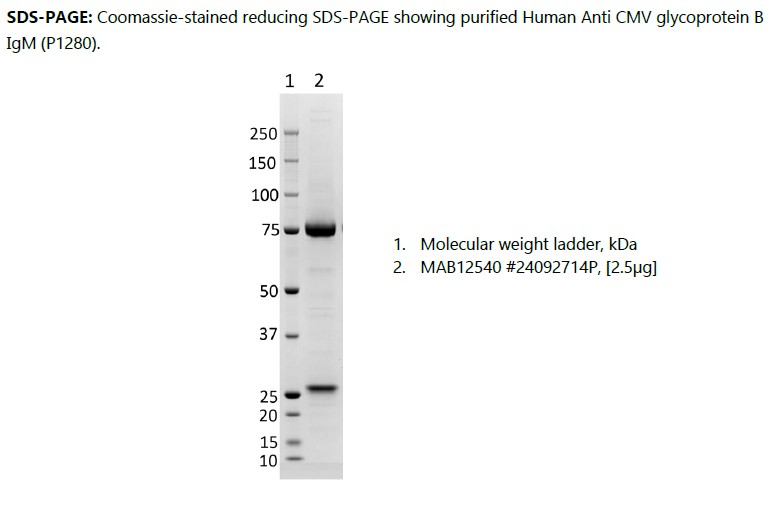
Human anti CMV glycoprotein B IgM (P1280), recombinant human IgM kappa, produced in HEK293 cells and purified by ion exchange chromatography and dialysis.
Human anti CMV glycoprotein B IgM (P1280), recombinant
Human anti CMV glycoprotein B IgM (P1280), recombinant human IgM kappa, produced in HEK293 cells and purified by ion exchange chromatography and dialysis
Human anti CMV glycoprotein B IgM (P1280), recombinant
- Recombinant Human IgM kappa
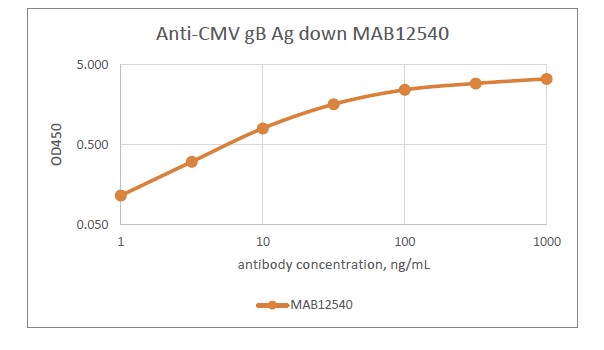
- This antibody is specific for human cytomegalovirus (HCMV) glycoprotein B (gB)
- Presented as Liquid, DPBS Buffer
BACKGROUND
Human cytomegalovirus (HCMV) is a prevalent virus and a significant global health challenge. Although no vaccine for HCMV has been licensed yet, research is increasingly focused on understanding the immune responses necessary to create effective vaccines. Glycoprotein B (gB), a crucial protein in HCMV’s infection process, is a promising target for vaccines and immunotherapies due to its essential role in viral entry into host cells. Studies suggest that vaccines based on recombinant gB can offer partial protection, underscoring the potential of antigen-specific strategies (Bootz et al., 2017).
Recent research has highlighted the importance of antibody functions beyond neutralization, such as antibody-dependent cellular cytotoxicity (ADCC), in managing HCMV infections. These non-neutralizing functions can also improve our understanding of immune protection, particularly in preventing vertical transmission from mother to child (d’Angelo et al., 2024).
Traditional methods like IgM and IgG testing can be prone to false positives, especially in high-risk cases such as pregnant women, where accurate diagnosis is critical for preventing congenital HCMV transmission (Weber et al., 2001). In contrast, recombinant antibodies offer enhanced sensitivity, specificity, and scalability, leading to more consistent and reliable diagnostic outcomes compared to human-derived plasma, which can vary in quality. This advancement significantly reduces the risk of false results, supporting both diagnostics and vaccine development efforts in controlling HCMV.
REFERENCES
- Bootz, A., Karbach, A., Spindler, J., Kropff, B., Reuter, N., Sticht, H., Winkler, T. H., Britt, W. J., & Mach, M. (2017). Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLOS Pathogens, 13(8), e1006601.
- d’Angelo, P., Zavaglio, F., Gabanti, E., Zelini, P., Fornara, C., Bernuzzi, S., Spinillo, A., Lilleri, D., & Baldanti, F. (2024). Evaluation of the In Vitro Capacity of Anti-Human Cytomegalovirus Antibodies to Initiate Antibody-Dependent Cell Cytotoxicity. Microorganisms, 12(7), 1355.
- Weber, B., Berger, A., & Rabenau, H. (2001). Human cytomegalovirus infection: Diagnostic potential of recombinant antigens for cytomegalovirus antibody detection. Journal of Virological Methods, 96(1), 67-79.