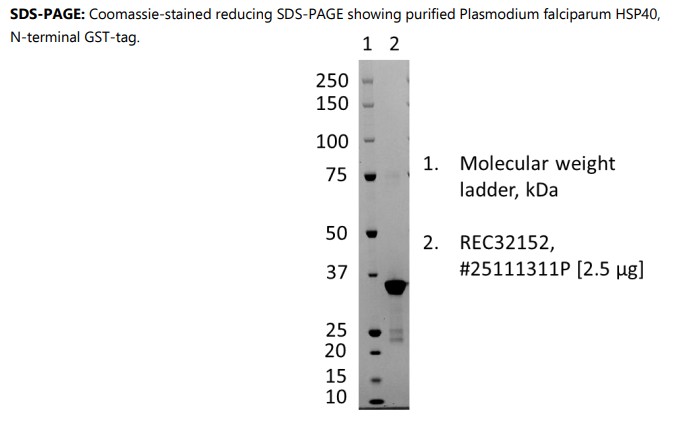
Plasmodium falciparum HSP40 protein
Price range: $310.00 through $1,317.50 excl. VAT
Recombinant Plasmodium falciparum HSP40, N-terminal GST-tag, produced as soluble protein in E. coli and purified by affinity chromatography, ion exchange chromatography and dialysis.
Plasmodium falciparum HSP40, Recombinant
Recombinant Plasmodium falciparum HSP40, N-terminal GST-tag, produced as soluble protein in E. coli and purified by affinity chromatography, ion exchange chromatography, and dialysis.
PRODUCT DETAILS – Plasmodium falciparum Etramp 5, Recombinant
- Accession: XP_001351570.2
- Tag: GST-tag, N-terminus
- Expressed in E.coli
- Presented as liquid in DPBS
BACKGROUND
Plasmodium falciparum Heat Shock Protein 40 (Hsp40) proteins form a diverse family of DnaJ‑type co‑chaperones that act with Hsp70 to maintain protein homeostasis during the parasite’s intra‑erythrocytic cycle, irrespective of whether they are classified as type I, II, or other sub‑types (Botha 2007; Shonhai 2007). All Hsp40s share a conserved J‑domain containing the HPD motif, which is essential for stimulating Hsp70 ATPase activity and thereby supporting protein folding, assembly, and protection against aggregation under physiological and stress conditions such as febrile temperatures (Shonhai 2007; Shonhai 2010).
Recombinant Hsp40 proteins from P. falciparum, together with their Hsp70 partners, are widely used as tools to study chaperone specificity and to explore parasite‑specific vulnerabilities for small‑molecule inhibition (Shonhai 2010; Botha 2007). In vaccine and sero‑epidemiological research, proteomic and multiplex serological studies have shown that Hsp40‑derived antigens can contribute to profiling antibody responses alongside classical blood‑stage and pre‑erythrocytic proteins, helping to refine diagnostic and vaccine candidate selection strategies in combination with other markers of exposure and protection (Molehin 2020; Alaerts 2025).
REFERENCES
- Botha, M., Pesce, E. R., & Shonhai, A. (2007). The Hsp40 proteins of Plasmodium falciparum and other apicomplexans. International Journal of Parasitology, 37(8–9), 831–841.
- Shonhai, A., Boshoff, A., & Blatch, G. L. (2007). The structural and functional diversity of Hsp70 systems in the malaria parasite Plasmodium falciparum. Protein Science, 16(9), 1803–1818.
- Shonhai, A. (2010). Plasmodial heat shock proteins: targets for chemotherapy. FEMS Pathogens and Disease, 58(1), 61–74.
- Molehin, A. J., et al. (2020). The use of proteomics for the identification of promising vaccine and diagnostic biomarkers in Plasmodium falciparum. Pathogens, 9(6), 477.
- Alaerts, M., Vermeulen, M., De Mulder, L., Van Den Bossche, D., Gyselinck, I., Cnops, L., Van Esbroeck, M., & Van Geertruyden, J. P. (2025). A multiplex serological assay to evaluate the antibody responses to Plasmodium falciparum antigens in different epidemiological settings. Vaccines, 13(11), 1091.