Plasmodium falciparum PfMSP1-19 protein
Price range: $310.00 through $1,317.50 excl. VAT
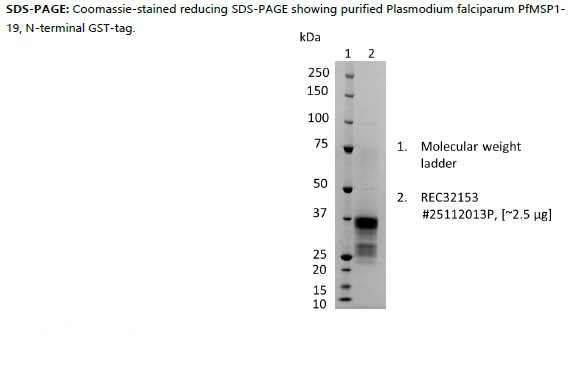
Recombinant Plasmodium falciparum PfMSP1-19, N-terminal GST-tag, produced in E.coli and purified by affinity chromatography, ion exchange chromatography and dialysis.
Plasmodium falciparum PfMSP1-19 protein, Recombinant
Recombinant Plasmodium falciparum PfMSP1-19, N-terminal GST-tag, produced in E.coli and purified by affinity chromatography, ion exchange chromatography, and dialysis.
PRODUCT DETAILS – Plasmodium falciparum Etramp 5, Recombinant
- Accession: AAK07641.1
- Tag: GST-tag, N-terminus
- Expressed in E.coli
- Presented as liquid in HBS: 50 mM HEPES, 150 mM NaCl, pH 8.0
BACKGROUND
Plasmodium falciparum Merozoite Surface Protein 1 (PfMSP1) is a major protein on the surface of the merozoite, the stage of the malaria parasite responsible for invading human erythrocytes. During the invasion process, MSP1 undergoes two major proteolytic cleavage events. The final product, a 19 kDa C-terminal fragment known as PfMSP1_19, remains anchored to the parasite membrane via a glycosylphosphatidylinositol (GPI) moiety and is the only portion of the MSP1 complex carried into the newly invaded erythrocyte (Blackman et al., 1990). This fragment consists of two tandem epidermal growth factor (EGF)-like domains that are highly conserved, playing a structural role that is vital for the parasite’s successful entry into the host cell.
As a recombinant protein, PfMSP1_19 serves as a primary candidate for blood-stage subunit vaccines and a robust tool for serological diagnostics. Its vaccine potential is centered on its ability to induce inhibitory antibodies that can interfere with parasite growth by inhibiting MSP1 processing, impairing merozoite invasion, and affecting intracellular parasite development (Moss et al., 2012). To ensure these recombinant versions are immunologically active, they must be produced under expression and purification conditions that support correct folding of all twelve cysteine residues into their native disulfide-bonded conformation, as demonstrated for PfMSP1_19 expressed in Escherichia coli (Chappel & Holder, 1993). Additionally, recombinant PfMSP1_19 is widely used in ELISA-based surveillance, allowing researchers to detect long-lived IgG antibodies in human populations and thereby measure malaria transmission intensity and historical exposure (Cook et al., 2010).
REFERENCES
-
- Blackman, M. J., Heidrich, H. G., Donachie, S., McBride, J. S., & Holder, A. A. (1990). A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. Journal of Experimental Medicine, 172(1), 379–382.
- Chappel, J. A., & Holder, A. A. (1993). Expression of the 19 kDa carboxyl-terminal fragment of Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Molecular and Biochemical Parasitology, 60(2), 303–311.
- Cook, J., Reid, H., Iavro, J., Kuwahata, M., Taleo, G., Clements, A., McCarthy, J., Vallely, A., & Drakeley, C. (2010). Using serological measures to monitor changes in malaria transmission in Vanuatu. Malaria Journal, 9, 169.
- Moss, D. K., Remarque, E. J., Faber, B. W., Cavanagh, D. R., Arnot, D. E., Thomas, A. W., & Holder, A. A. (2012). Plasmodium falciparum 19-kilodalton merozoite surface protein 1 (MSP1)-specific antibodies that interfere with parasite growth in vitro can inhibit MSP1 processing, merozoite invasion, and intracellular parasite development. Infection and Immunity, 80(3), 1280–1287.