ELISA: ELISA detection of IgG and IgM antibodies to spike glycoprotein (E1), nucleoprotein (NP) and virus-like particles (VLP) of Rubella virus.
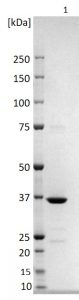
SDS-PAGE: Reducing SDS-PAGE gel showing purified Rubella virus nucleoprotein, showing protein migrating as a band of approximately 36kDa.
RUBELLA VIRUS NUCLEOPROTEIN (STRAIN F-THERIEN)
Rubella Virus Nucleoprotein is produced by recombinant expression of Rubella structural polyprotein (amino acids 1-300, NCBI accession number NP_062884.1) in HEK293 cells. It contains a C-terminal 10 amino acid glycine-serine linker followed by a 6x histidine tag.
PRODUCT DETAILS – RUBELLA VIRUS NUCLEOPROTEIN (STRAIN F-THERIEN)
- Recombinant Rubella virus Nucleoprotein expressed from HEK293 cells (strain F-Therien, NCBI Accession Number: U69551.1).
- Includes amino acids 1-300 and a C-terminal, 10 amino acid glycine-serine linker followed by a His-tag.
- Greater than 95% purity by SDS-PAGE and buffered in 20mM TRIS-HCl pH8.0, 50mM NaCl, 0.5% SDS.
BACKGROUND
Rubella virus is an enveloped, positive single-stranded RNA virus and a member of the genus Rubivirus, which belongs to the Togaviridae family. The structural proteins of Rubella virus are synthesized as a polyprotein precursor in association with the endoplasmic reticulum in the host cell. It consists of three structural proteins: a capsid protein and two membrane-spanning glycoproteins, E1 and E2, localized in the virus envelope (Oker-Blom, et al., 1983). The polyprotein is cotranslationally cleaved by the host-cell signal peptidase and the C terminus of the capsid protein remains attached to the E2 signal peptide, which anchors this viral protein to intracellular membranes (Suomalainen, et al., 1990).
The capsid protein, excluding the E2 signal peptide, is a 277-amino-acid residue protein that forms disulfide-linked dimers (Baron & Forsell, 1991). It interacts with the RNA genome and forms the nucleocapsid, which is surrounded by a lipid membrane upon which E1 and E2 are arranged (Hobman & Chantler, 2007). The capsid protein is an essential component of the virus and a key factor for successful replication of the virus in host cells (Mangala Prasad, et al., 2013). It not only packages the RNA genome but also is involved in viral transcription and replication, regulating viral RNA replication by interacting with the virus-encoded nonstructural proteins (Chen & Icenogle, 2004).
Crystal structures of the C-terminal part of the protein (amino acids 127–277) suggest that variability in the relative positions of the secondary structure elements may contribute to flexibility in the capsid protein structure; this flexibility may be required for other non-structural cellular functions to facilitate virus replication and assembly inside host cells (Ilkow, et al., 2010; Mangala Prasad, et al., 2013).
REFERENCES
- Baron, M. D. & Forsell, K., 1991. Oligomerization of the structural proteins of rubella virus. Virology, 185(2), pp. 811-819.
- Chen, M. H. & Icenogle, J. P., 2004. Rubella virus capsid protein modulates viral genome replication and virus infectivity. J Virol., 78(8), p. 4314–4322.
- Hobman, T. C. & Chantler, J., 2007. Rubella virus. In: D. M. Knipe & P. M. Howley, eds. Fields Virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, p. 1069–1100.
- Ilkow, C. S., Willows, S. D. & Hobman, T. C., 2010. Rubella virus capsid protein: A small protein with big functions. Future Microbiol., 4(571–584), p. 5.
- Mangala Prasad, V. et al., 2013. Rubella virus capsid protein structure and its role in virus assembly and infection. Proc Natl Acad Sci U S A, 110(50), p. 20105–20110.
- Oker-Blom, C., Kalkkinen, N., Kääriäinen, L. & Pettersson, R., 1983. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J. Virol., Volume 964–973, p. 964–973.
- Suomalainen, M., Garoff, H. & Baron, M. D., 1990. The E2 signal sequence of rubella virus remains part of the capsid protein and confers membrane association in vitro. J Virol. , 64(11), p. 5500–5509.