By Holge Hannemann, Director of Research, The Native Antigen Company
Influenza viruses A and B are a major public health concern, mainly affecting the pharynx, trachea, and sometimes the lungs of millions of people worldwide each year, with symptoms that range from mild to life-threatening. Alongside seasonal outbreaks (which tend to prevail over the winter months) there is also the looming threat of pandemic influenza strains, which have the potential to bring healthcare systems to a standstill because most people have little or no immunity. The most effective preventative measure against flu is vaccination. Here, I’ll explore the challenges associated with flu vaccine development – and the emerging technologies hoping to tackle them.
THE CURRENT VACCINE LANDSCAPE
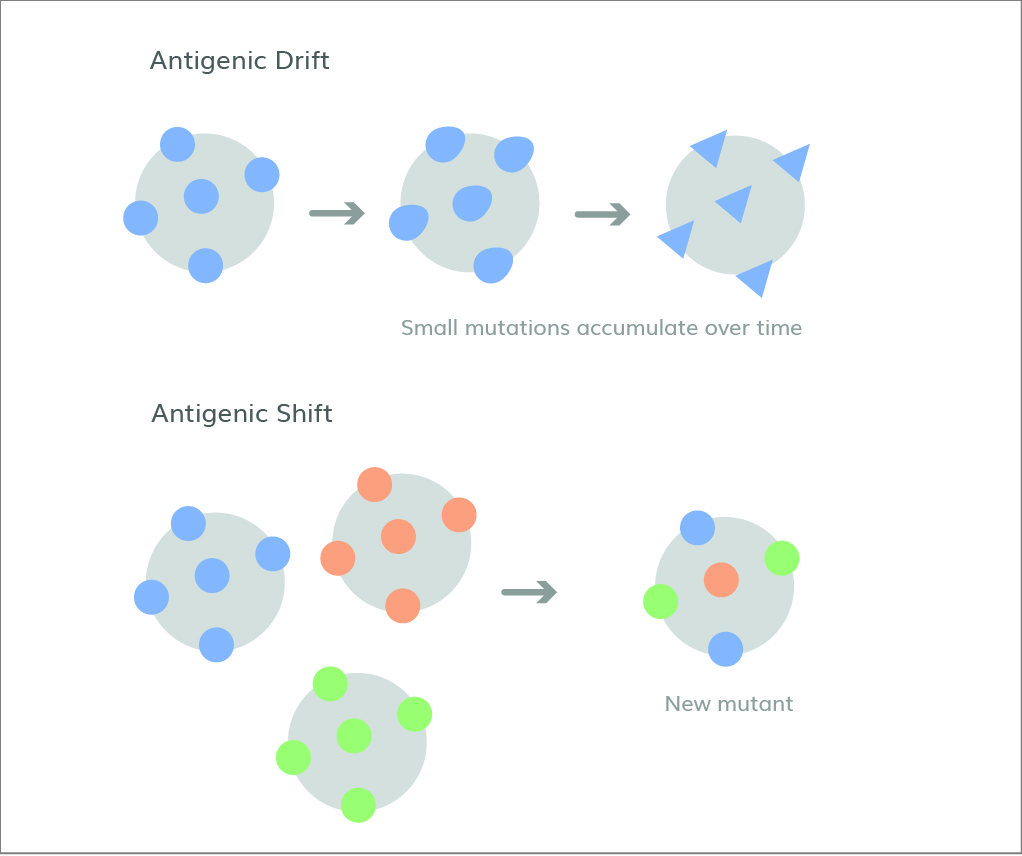
Figure 1. Antigenic drift is the gradual accumulation of smaller mutations, whereas antigenic shift is a sudden, drastic change.
Most influenza vaccines elicit antibodies against the major viral surface proteins, hemagglutinin (HA) and neuraminidase (NA). This poses a challenge for vaccine formulation because evolutionary changes in these proteins enable the virus to evade the adaptive immune response through a combination of phenomena known as antigenic drift and shift (1), as shown in Figure 1. Antigenic drift occurs when influenza viruses continuously undergo changes to their HA and NA surface proteins through mutation. These changes accumulate over time, transforming the viruses and allowing them to escape host recognition without hindering their ability to gain entry to cells. Antigen shift, on the other hand, is much rarer and is defined by abrupt changes to HA and NA through genome reassortment by influenza A with other influenza subtypesthat are co-infecting the same host cell, made possible by the segmented nature of the influenza virus genome. These drastic changes allow influenza viruses to catch our immune systems completely off-guard and rapidly spread through populations, leading to pandemics. The 1918 Spanish Flu and 1968/1969 flu pandemic are two notable historical examples of this. As a result, vaccine formulations must be regularly updated to keep up with emerging forms of the flu viruses.
Standard flu vaccines are formed from inactivated and liveattenuated viruses produced using chicken eggs – every year, vaccine producers consume millions of eggs in this process. Although this is the current leading strategy, it is a highly complex and time-consuming process, typically taking 6–8 months to produce sufficient quantities. Because of the long timescales involved, the WHO meets twice yearly to predict and select the vaccine components for the upcoming influenza seasons in the Northern and Southern Hemispheres. These predictions inform the entire vaccine development chain – from the reagents used for research and development through to selecting formulations and manufacturing the vaccines themselves. Unfortunately, despite these efforts, vaccine efficacy is usually under 60 percent – and can even drop as low as 10 percent (2). Uncertainties in strain prediction can result in vaccines that significantly differ from the strain circulating for that season (3).
Because vaccines play such a vital role in preventing serious flu outbreaks, the challenges in their development and production pose a real threat to human health. This risk is exacerbated during pandemics, as we saw in the devastating 2009 Swine Flu outbreak – during which a delayed vaccine roll-out is thought to have caused thousands of additional deaths (4). Consequently, there has been a significant push within the industry for more sustainable and effective alternatives. One avenue currently being explored is a universal flu vaccine. A successful universal vaccine would ideally eliminate the need for seasonal re-formulation by providing robust, long-lasting protection against multiple subtypes of flu and emerging strains, as opposed to just a select few. A popular target for universal vaccines is HA’s stalk domain, which plays a fundamental role in membrane fusion. Unlike the head of the HA protein, the stalk typically remains unchanged and the elicited antibodies tend to ward off a variety of other strains (5). A number of candidates are in development, with various approaches being taken.
ALL EYES AHEAD, ALL EYES ON THE BALL…
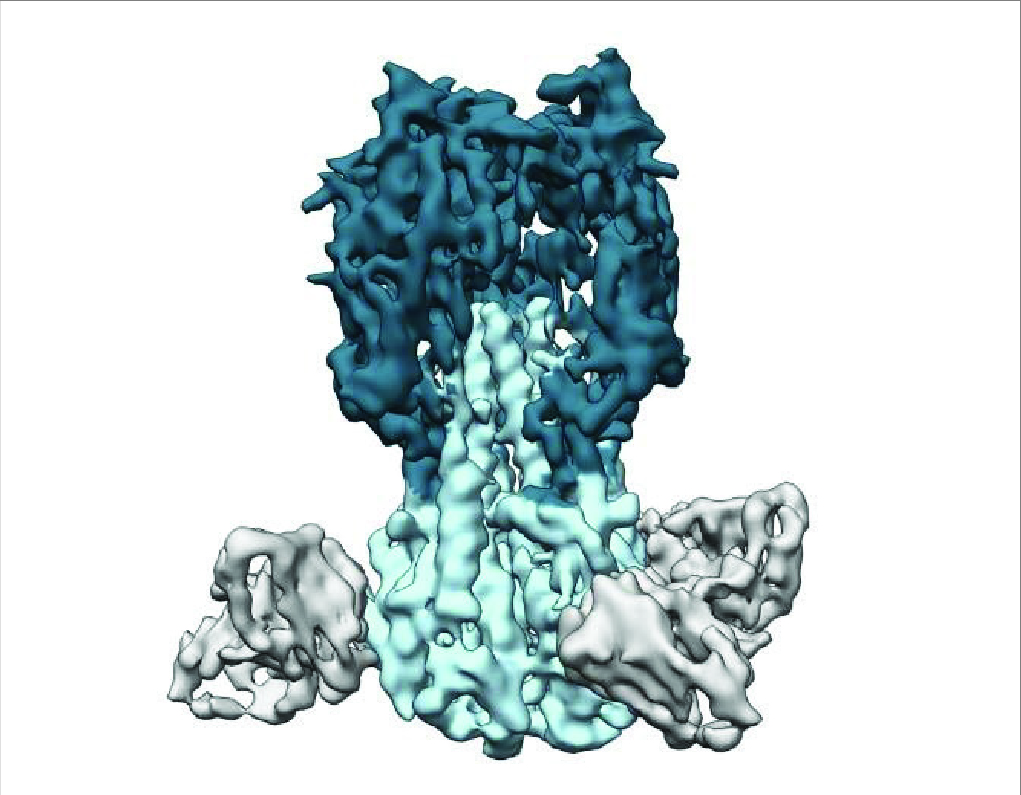
Figure 2. Traditional seasonal flu vaccines target the “head” of the HA protein, whereas universal vaccines trigger antibodies (partially shown in gray) that bind to the stalk (light blue) (7).
Although they are now famous thanks to Moderna and Pfizer-BioNTech, mRNA vaccines had been in development as alternatives to live attenuated/inactivated virus/recombinant protein vaccines for a while before the SARS-CoV-2 pandemic hit. The technology works by delivering mRNA to cells that subsequently express HA proteins, which the body can then produce antibodies against. We have seen how successful the platform can be, allowing researchers to quickly swap mRNA encoding for different antigens. This technology holds great promise for influenza because it provides a means to rapidly change the vaccine target without repeated approval procedures, and thus use highly modular technology and manufacturing to hedge bets against and rapidly respond to emerging and potential pandemic strains. Other benefits of mRNA vaccines include a shorter manufacturing period than traditional egg-based methods, and greater potential efficacy. Combining this technology with universal vaccine approaches could be key to overcoming the challenges of season flu and erasing the threat of pandemics.
A team of researchers at the University of Pennsylvania is using mRNA technology to test a universal vaccine that contains HA proteins from 20 different flu lineages (6). Studies in mice and ferrets have shown promising results in reducing signs of illness and protecting against death, even when exposed to strains not included in the vaccine. Though these new technologies present exciting developments and possibilities for the future, it is also important to focus on improving existing vaccines and create solutions that can be immediately implemented. Next-generation adjuvants could provide answers by enhancing and modulating the immune response. Adjuvants are substances that boost immune response through various mechanisms – including the creation of antigen depots, the activation of the innate immune response, the recruitment of immune cells, and improvements in antigen
uptake. Currently there are only six licensed adjuvants available in combination with flu vaccine: Alum, MF59, AS03, AF03, virosomes, and heat labile enterotoxin. Thus, there is much space yet to explore. The discovery and design of novel adjuvants could be particularly helpful for inducing robust antibody responses in high-risk populations, such as the elderly or those with pre-existing conditions.
Ultimately, emerging technologies offer a great deal of promise in tackling flu – and the COVID-19 pandemic has already demonstrated our ability to rapidly deploy some of these with real success. To continue along this trajectory, reagent companies, like my own, must continue to support vaccine developers and manufacturers by providing the very latest high-quality products to facilitate efficient R&D. We do this by regularly updating our supplies according to the latest WHO predictions, ensuring researchers have access to relevant recombinant proteins as quickly as possible. Such cooperation and communication will enable mankind to continue to strengthen existing and new approaches for flu vaccine development. Through improved strain prediction and disease surveillance, we will be able to build a comprehensive defense against the moving target that is influenza.
References 1. S Couma, EM Anderson, SE Hensley, Annual Review of Virology, 7, 495-512 (2020). DOI: 10.1146/annurev-virology-010320-044746 2. CDC, “Past Seasons’ Vaccine Effectiveness Estimates,” (2022). Available at https://bit.ly/3jinseM 3. Bill & Melinda Gates Foundation, “Ending the Pandemic Threat: A Grand Challenge for Universal Influenza Vaccine Development,” (2018). Available at https://bit.ly/2HwEI7F 4. HV Fineberg, N Engl J Med, 270, 1335-1342 (2014). DOI: 10.1056/ NEJMra1208802 5. NIAID, “Universal Influenza Vaccine Research,” (2019). Available at https://bit.ly/3l4OlTW 6. NIH, “Using mRNA technology for a universal flu vaccine,” (2022). Available at https://bit.ly/3jjNluF 7. Science, “Innovative universal flu vaccine shows promise in first clinical test,” (2020). Available at https://bit.ly/3YaiYFS

