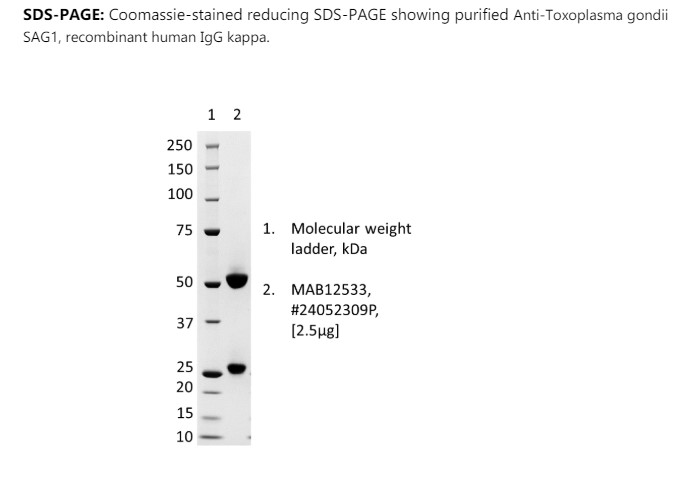
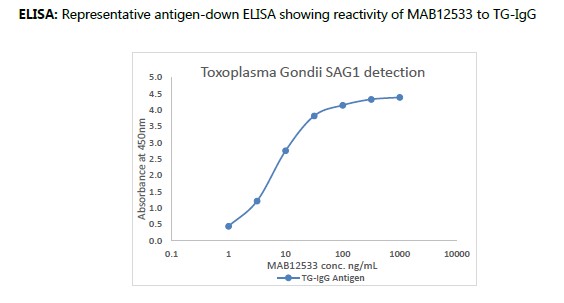
Anti-Toxoplasma gondii SAG1, recombinant human IgG kappa
Price range: $346.02 through $1,470.57 excl. VAT
Anti-d SAG1, recombinant human IgG kappa, produced in HEK293 cells and purified by affinity chromatography and dialysis
Anti-Toxoplasma gondii SAG1, recombinant human IgG kappa
Anti-d SAG1, recombinant human IgG kappa, produced in HEK293 cells and purified by affinity chromatography and dialysis
Anti-Toxoplasma gondii SAG1, recombinant human IgG kappa
- Recombinant human IgG kappa
- This antibody is specific for major surface protein SAG-1 (p30) of Toxoplasma gondii (T.gondii)
- Presented as Liquid, DPBS Buffer
BACKGROUND
Toxoplasma gondii (T. gondii) is an obligate intracellular parasitic protozoan of the phylum Apicomplexa. It is the causative agent of the disease toxoplasmosis, a common parasitic zoonosis which is widespread throughout most of the world. The domestic cat and other members of the family Felidae are the only definitive hosts for T.gondii. However, T.gondii is also capable of infecting a wide range of birds and mammals, including humans, which act as intermediate hosts.
In domestic cats, sexual replication of T. gondii occurs with the release of oocysts into the environment, in cat faeces. In humans, T. gondii is primarily acquired by ingesting undercooked meat contaminated with bradyzoites (tissue cysts), drinking water contaminated by oocysts, or via accidental ingestion of cat faeces containing oocysts. In pregnant women, infected with T. gondii, vertical transmission of the parasite can occur causing congenital defects, stillbirths or miscarriage. In rare cases, infection via blood transfusion and organ transplant can also occur (CDC).
Antibodies play a pivotal role in both the diagnosis and potential therapeutic applications for toxoplasmosis. The detection of IgG antibodies against T. gondii can indicate past or ongoing infection, and their avidity can help distinguish between acute and chronic phases of the disease (Beghetto et al., 2006; Holec-Gąsior et al., 2010).
Utilizing recombinant Toxoplasma gondii antibodies in serological assays enhances sensitivity and lowers the risk of false positives and negatives. These antibodies serve as effective substitutes for high-titer positive human plasma in producing calibrators and controls for diagnostic assays, ensuring improved reproducibility, flexibility, and scalability (Hackett et al., 1998). This leads to better detection and monitoring of T. gondii infections, facilitating timely and accurate diagnosis and improving disease management and control efforts.
REFERENCES
- CDC (Centers for Disease Control and Prevention): Parasites – Toxoplasmosis (Toxoplasma infection)
- Beghetto, E., Spadoni, A., Bruno, L., Buffolano, W., & Gargano, N. (2006). Chimeric Antigens of Toxoplasma gondii: Toward Standardization of Toxoplasmosis Serodiagnosis Using Recombinant Products. Journal of Clinical Microbiology, 44(6), 2133-2140.
- Holec-Gąsior, L., Drapała, D., Lautenbach, D., & Kuri, J. (2010). Toxoplasma gondii: Usefulness of ROP1 Recombinant Antigen in an Immunoglobulin G Avidity Assay for Diagnosis of Acute Toxoplasmosis in Humans. Polish Journal of Microbiology, 59(4), 307-310.
- Hackett, J. Jr, Hoff-Velk, J., Golden, A., Brashear, J., Robinson, J., Rapp, M., Klass, M., Ostrow, D. H., & Mandecki, W. (1998). Recombinant Mouse-Human Chimeric Antibodies as Calibrators in Immunoassays that Measure Antibodies to Toxoplasma gondii. Journal of Clinical Microbiology, 36(5), 1277-1284. https://doi.org/10.1128/JCM.36.5.1277-1284.1998