BIOTIN LABELING KIT
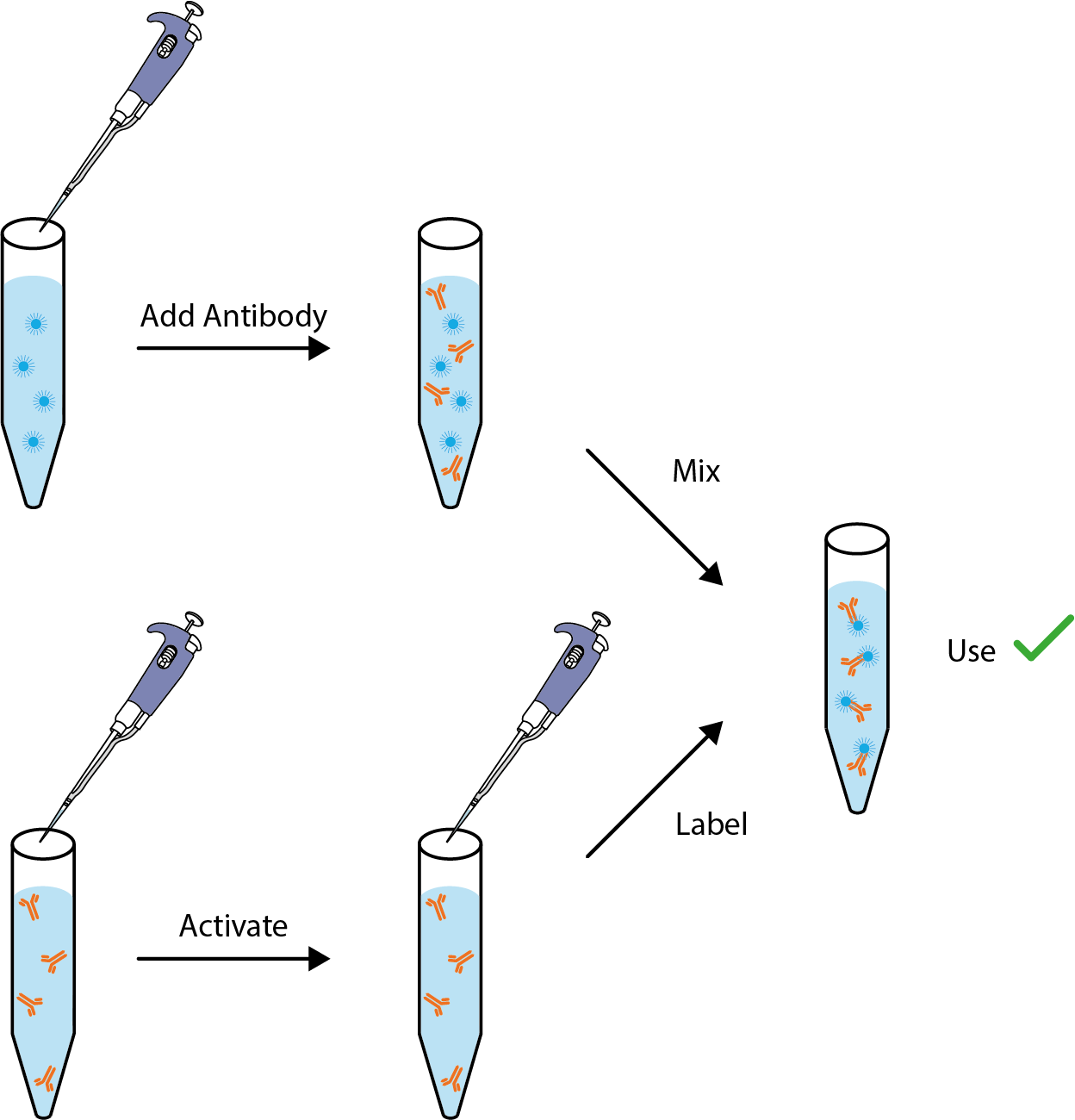
The Biotin Labeling kit utilizes a novel chemistry to generate highly reproducible biotinylated conjugates with a simple procedure. Manufacturing quality systems ensure excellent sensitivity, low backgrounds and lot to-lot consistency. Each kit includes all reagents needed to biotinylate the stated amount of IgG and includes a highly pure solution of 100 mg/ml BSA for final formulation.
Kits are available for labeling 0.3mg, 1mg and 10mg IgG.
PRODUCT DETAILS – BIOTIN LABELING KIT
- Can be used with up to 10 mg/ml (1%) BSA as a carrier protein.
- Reaction complete in as little as 10 minutes.
- Room temperature-stable active biotinylation reagent.
- Pre-measured active biotinylation reagent coated onto a tube surface.
- Completely scalable: conjugate anywhere from 0.1 to 1 gram IgG per reaction.
- Highly efficient Biotin incorporation.
- Purification not usually required.
- Shelf life of 24 to 48 months.
- No license needed for commercial use.
BACKGROUND
The Biotin Labeling Kit uses a simple and fast process to conjugate an antibody to Biotin. Biotinylation is the process of covalently attaching biotin to molecules and is rapid, specific and due to the small size of biotin (MW = 244.31 g/mol), is unlikely to disturb the natural function of the molecule. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited to isolate and detect biotinylated molecules of interest. For example, Biotin can be used for detection via anti-biotin antibodies or avidin/streptavidin-tagged detection strategies such as enzyme reporters (e.g., horseradish peroxidase, alkaline phosphatase) or fluorescent probes. This can be useful in localization by fluorescent or electron microscopy, ELISA assays, ELISPOT assays, western blots and other immunoanalytical methods. For example, biotin-labeled antibodies have been used to develop sandwich ELISA for detection of Leishmania antigens (Zhang et al., 2019), cross-competition assays to determine if antibodies recognise overlapping or non-overlapping sties on Dengue virus Envelope protein (Schieffelin et al., 2010) and binding assays with Zika virus Envelope protein (Zhao et al., 2016).
REFERENCES
- Schieffelin et al. (2010). Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virology Journal volume 7, Article number: 2.
- Zhang et al. (2019). Development of a sandwich ELISA to detect Leishmania 40S ribosomal protein S12 antigen from blood samples of visceral leishmaniasis patients. BMC Infect Dis. 18: 500.
- Zhao et al. (2016). Structural basis of Zika virus specific antibody protection. Cell. 166(4): 1016–1027.