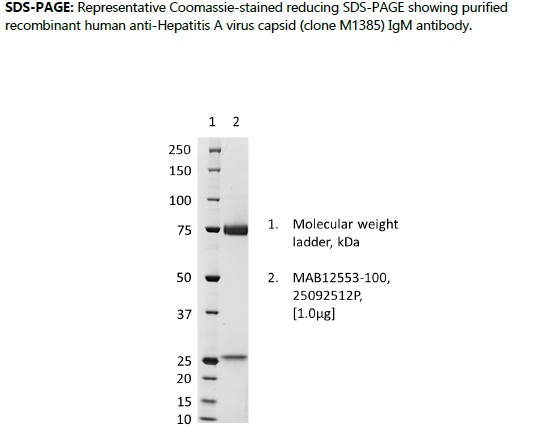
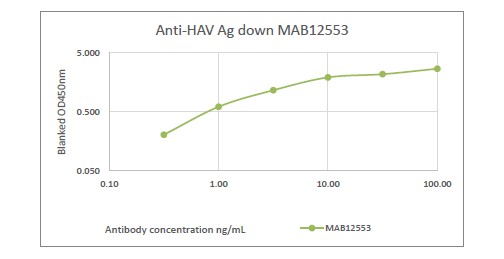
Human anti-Hepatitis A virus capsid IgM (clone M1385), Recombinant
Recombinant human anti-Hepatitis A virus capsid (clone M1385) IgM antibody, produced in HEK293 cells and purified by ion exchange chromatography, ammonium sulphate precipitation and dialysis.
PRODUCT DETAILS – Human anti-Hepatitis A virus capsid IgM (clone M1385)
- Isotype: human IgM
- Clone Number: M1385
- Presented as Liquid in DPBS Buffer.
Background
Hepatitis A, caused by the Hepatitis A virus (HAV), continues to present a significant global health burden, spreading primarily through the fecal–oral route via contaminated food and water (CDC, 2020; World Health Organization, 2022). Although often a self-limiting liver infection, the potential for severe complications, including acute liver failure in rare cases, underlines the importance of accurate HAV diagnostics and effective surveillance programs. Serological testing remains the cornerstone of Hepatitis A diagnosis, with detection of HAV-specific antibodies providing essential clinical insights: IgM antibodies indicate acute infection, while IgG antibodies confirm immunity, either from past exposure or vaccination (Jacobsen & Wiersma, 2016). These results are critical not only for patient management but also for informing vaccination strategies and monitoring population-level immunity.
The future of reliable Hepatitis A testing lies in the adoption of recombinant antibodies, which are engineered using advanced recombinant DNA methods to target specific HAV immunogenic proteins, particularly the capsid proteins VP1, VP2, and VP3 (Lin et al., 2005; Song et al., 2011). Unlike conventional antibodies derived from human or animal serum, recombinant anti-HAV antibodies deliver defined specificity, enhanced purity, and high batch-to-batch consistency. These qualities reduce variability in serological assays and ensure reproducible diagnostic results across laboratories and manufacturers. As demand for high-quality reagents continues to rise, recombinant antibodies are increasingly recognized as indispensable tools for advancing HAV diagnostics, particularly in contexts where reliability and standardization are important.
In clinical practice, recombinant anti-HAV IgG antibodies play a central role as calibrators, controls, and critical components in widely used platforms such as ELISA (Fujiwara et al., 2017). Their application allows laboratories to determine immune status with precision, detect evidence of past Hepatitis A infection, and evaluate vaccine efficacy with confidence. By improving the consistency and reliability of HAV diagnostic assays, these DNA-engineered reagents strengthen global surveillance efforts, enhance the quality of public health data, and support more effective control programs. Well-characterized recombinant HAV antibodies and antigens are therefore essential not only for assay performance and validation but also for sustaining robust public health strategies in the fight against Hepatitis A (WHO, 2022).
REFERENCES
CDC. (2020). Hepatitis A Information. Centers for Disease Control and Prevention.
Fujiwara, K. et al. (2017). Development of an enhanced immunoassay for detecting total antibody to hepatitis A virus. Journal of Virological Methods, 243, 117-123.
Jacobsen, K. H., & Wiersma, S. T. (2016). Hepatitis A virus seroprevalence in the USA: a systematic review. Vaccine, 34(34), 3922-3928.
Lin, S. T. et al. (2005). Construction and expression of recombinant hepatitis A virus VP1 protein in Escherichia coli. Journal of Virological Methods, 129(1), 1-8.
Song, Q. et al. (2011). Preparation and characterization of monoclonal antibody against hepatitis A virus capsid protein VP1. Hybridoma (Larchmt), 30(2), 173-178.