Influenza A/Massachusetts/18/2022 (H3N2)-like virus neuraminidase (tetrameric)
Price range: $908.29 through $3,860.23 excl. VAT
Influenza A/Massachusetts/18/2022 (H3N2)-like neuraminidase, N-terminal His-tetrabrachion domain, produced in HEK293 cells and purified by affinity chromatography and dialysis.
Influenza A/Massachusetts/18/2022 (H3N2)-like virus neuraminidase (tetrameric)
Influenza A/Massachusetts/18/2022 (H3N2)-like neuraminidase, N-terminal His-tetrabrachion domain, produced in HEK293 cells and purified by affinity chromatography and dialysis.
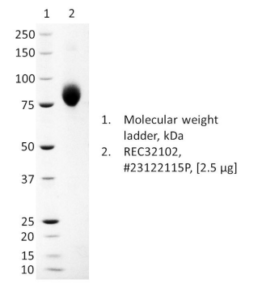
PRODUCT DETAILS – Influenza A/Massachusetts/18/2022 (H3N2)-like virus neuraminidase (tetrameric)
- Recombinant Influenza A/Massachusetts/18/2022 (H3N2) strain
- GISAID: EPI2413618
- Recombinant protein manufactured in HEK293 cells
- Purified from supernatant by immobilized metal affinity chromatography and buffer exchange.
- Presented as liquid in DPBS with high purity by SDS-PAGE.
BACKGROUND
The influenza A viruses are negative-sense, single-stranded, segmented RNA viruses of the genus Alphainfluenzavirus, family Orthomyxoviridae. There are several subtypes, named according to the type of Haemagglutinin (H1-18) and Neuraminidase (N1-11) (Centers for Disease Control and Prevention, 2017). Humans are generally infected by influenza viruses of the subtypes H1, H2 or H3, and N1 or N2. In April 2009, a new virus, referred to as A/(H1N1) pdm09, appeared in Mexico and California (US), and was responsible for the first pandemics of the 21st century (claiming several hundred lives). It spreads rapidly from person to person, and is not related to any circulating inter-pandemic viruses. It is a quadruple reassortant virus, consisting of two swine-origin viruses, one avian-origin virus and one human-origin virus. It has a high fatality rate and shows higher incidence among younger people (Baldo et al., 2016). WHO convenes technical consultations in February and September each year to recommend viruses for inclusion in influenza vaccines for the northern and southern hemisphere influenza seasons, respectively.
Globally, from September 2020 through January 2021, overall influenza A virus detections were in the minority during this period compared to influenza B virus detections. However, from September to November 2020, influenza A virus detections predominated in some countries. In most countries, areas and territories reporting influenza A viruses, both A(H1N1)pdm09 and A(H3N2) subtypes were detected. WHO has identified four strains as the most likely to circulate in the 2022-23 influenza season, including Influenza A/Victoria/2570/2019 (H1N1)pdm09-like virus (WHO, 2022).
Since December, 2019, coronavirus disease 2019 (COVID-19) has been an international public health emergency and co-infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses have been reported, complicating their diagnosis (Azekawa et al., 2020; Cuadrado-Payán et al., 2020; Wu et al., 2020).
REFERENCES
-
- Azekawa S, Namkoong H, Mitamura K, Kawaoka Y, Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20:e00775. Published 2020 Apr 21.
- Baldo V, Bertoncello C, Cocchio S, et al. The new pandemic influenza A/(H1N1)pdm09 virus: is it really “new”?. J Prev Med Hyg. 2016;57(1):E19-E22.
- Centers for Disease Control and Prevention. (2017). Influenza Type A Viruses.
- Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84.
- WHO. Recommended composition of influenza virus vaccines for use in the 2022-2023 northern hemisphere influenza season. 2022.
- Wu X, Cai Y, Huang X, et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg Infect Dis. 2020;26(6):1324-1326.

![Influenza A [A/Hong Kong/483/97 (H5N1)] Hemagglutinin (HA), His-Tag](https://thenativeantigencompany.com/wp-content/uploads/1970/01/shutterstock_1204546204-300x300.jpg)