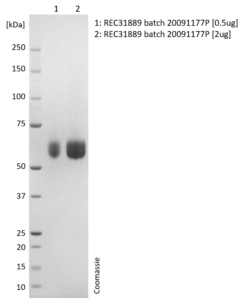
SDS-PAGE: Coomassie-stained reducing SDS-PAGE showing purified Influenza B Neuraminidase (NA) protein.
Influenza B [B/Washington/02/2019] Neuraminidase (NA), His-Tag
$553.29 – $1,939.44 excl. VAT
A recombinant influenza virus neuraminidase protein derived from the NA sequence of Influenza B/Washington/02/2019, expressed and purified from HEK293 cells as aa 44-466, and fused with a polyhistidine tag at the C-terminus. Manufactured in HEK293 cells to This virus is recommended by WHO for inclusion in the quadrivalent and trivalent vaccines for use in the 2020 – 2021 northern hemisphere influenza season.
INFLUENZA B [B/WASHINGTON/02/2019] NEURAMINIDASE (NA), HIS-TAG
This Influenza virus neuraminidase protein is derived from the NA sequence of B/Washington/02/2019, expressing aa 44-466, and fused with a polyhistidine tag at the C-terminus. The influenza virus neuraminidase protein is expressed in HEK293 cells. This virus is recommended by WHO for inclusion in the quadrivalent and trivalent vaccines for use in the 2020 – 2021 northern hemisphere influenza season.
PRODUCT DETAILS – INFLUENZA B [B/WASHINGTON/02/2019] NEURAMINIDASE (NA), HIS-TAG
- Recombinant Influenza B [B/Washington/02/2019] NA (NCBI Accession Number: QDJ74344.1), amino acids 44-466 and a C-terminal His-tag.
- Expressed in HEK293 cells, and purified from culture supernatant by immobilised metal affinity chromatography and buffer exchange.
- Presented as liquid in DPBS and greater than >95% purity by SDS-PAGE.
BACKGROUND
Influenza B virus is the only species in the genus Betainfluenzavirus in the virus family Orthomyxoviridae. Influenza B virus is only known to infect humans and seals and this is believed to account for the lack of associated influenza pandemics in contrast with those caused by the morphologically similar influenza A virus, although both mutate by both antigenic drift and reassortment. However, it is thought that Influenza B virus could cause significant morbidity and mortality worldwide, particularly in adolescents and schoolchildren. WHO convenes technical consultations in February and September each year to recommend viruses for inclusion in influenza vaccines for the northern and southern hemisphere influenza seasons, respectively. Flu vaccines are based on predicting which mutants of H1N1, H3N2, H1N2, and influenza B will proliferate in the next season. Separate vaccines are developed for the Northern and Southern Hemispheres in preparation for their annual epidemics. One influenza A (H1N1), one influenza A (H3N2), and one or two influenza B viruses (depending on the vaccine) are included in each season’s influenza vaccines (CDC, 2019). WHO has identified four strains as the most likely to circulate in the 2020-2021 influenza season, including Influenza B [B/Washington/02/2019] virus. Since December, 2019, coronavirus disease 2019 (COVID-19) has been an international public health emergency and co-infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses have been reported, complicating their diagnosis (Azekawa et al., 2020; Cuadrado-Payán et al., 2020; Wu et al., 2020).
REFERENCES
-
- Azekawa S, Namkoong H, Mitamura K, Kawaoka Y, Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20:e00775. Published 2020 Apr 21.
- Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84.
- Wu X, Cai Y, Huang X, et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg Infect Dis. 2020;26(6):1324-1326.
- Centers for Disease Control and Prevention (CDC). (2019). 1968 Pandemic (H3N2 virus).
- World Health organisation (WHO). Influenza. Recommended composition of influenza virus vaccines for use in the 2020 – 2021 northern hemisphere influenza season (2020).