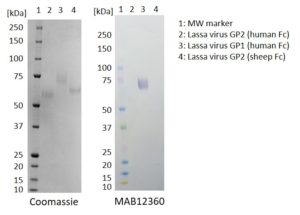
Western Blot: 100ng of each antigen (Lassa virus GP2 (human Fc), GP1 (human Fc) and GP2 (sheep Fc)) was used for SDS-PAGE, in reduced form. Proteins were transferred using Transblot for 7 minutes at 25V. 5% dry milk in PBS-T was used as blocking buffer and dilution buffer for antibodies. Primary antibodies (MAB12359, MAB12360 and MAB12361), and goat anti-mouse-IgG-HRP secondary antibody was used at 1:1000. All steps were carried out for 1h at room temperature with gentle rocking. KPL Membrane TMB was used for detection. Development time 1 minute.
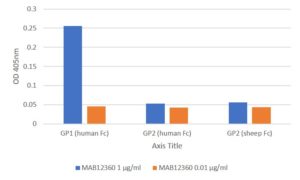
ELISA: All antigens coated at 0.5µg/ml in DPBS overnight at 2-8C. Plate washed 1 X 300µl/well TBS + 0.1% Tween20, blocked 300µl/well DPBS+1% BSA for an hour. Detection antibody diluted to 1.0µg/ml and 0.01µgml in DPBS + 1% BSA + 0.05% T20 and added at 100µl/well, incubated shaken 2h room temperature. Plate washed 3 X 300µl/well TBS-T wash buffer. Secondary antibody Biorad goat anti-mouse IgG-HRP (103005) diluted 1 in 2500 in DPBS/1%BSA/0.05%T20, added at 100µl/well, incubated shaken 1h room temperature. Plate washed 6X 300µl/well TBS-T wash buffer. Detection reagent Europa TMB substrate added at 100µl/well and the plate developed for 1.5 min. static on the bench. Reaction stopped with 100µl/well 1M HCL and the plate was read within 5 min. at 405nm.
MOUSE ANTI-LASSA FEVER VIRUS GP1 (HD7)
Mouse anti Lassa Fever virus GP1 antibody (clone HD7) is specific for Lassa Fever virus glycoprotein GP1 in western blot and ELISA and shows no cross-reactivity with GP2. The antibody is suitable for Western blot and ELISA applications.
PRODUCT DETAILS – MOUSE ANTI-LASSA FEVER VIRUS GP1 (HD7)
- Mouse anti Lassa Fever virus GP1 antibody (clone HD7) monoclonal antibody.
- Suitable for use in ELISA and Western blot applications. Antibody does not neutralise Lassa pseudoviruses in pMN assay.
- Purified by Protein G Sepharose chromatography.
- Presented in phosphate buffered saline, pH 7.4.
BACKGROUND
Lassa fever is a severe and sometimes fatal haemorrhagic disease caused by the Lassa fever virus (LAFV). First identified in 1969 in Nigeria, Lassa Fever virus is a single-stranded, enveloped RNA virus that belongs to the genus Mammarenavirus, of the Arenaviridae family of viruses. The natural reservoir for Lassa fever virus is the Mastomys natalensis rat and transmission of LAFV to humans most commonly occurs through contaminated rat urine and faeces. The virus is also spread by person-to-person via contact with contaminated human excreta, blood and bodily secretions, which causes a significant risk to health workers (1). LAFV is now endemic in West Africa and a substantial outbreak of Lassa fever in 2016 resulted in 160 deaths.
Lassa virus (LASV) is an enveloped, single-stranded RNA virus belonging to the arenavirus family. Arenavirus has more than 30 members divided into two groups: the New World viruses (or Tacaribe complex) and the Old World viruses (or LCM-Lassa complex). The New World family mainly contains Venezuelan hemorrhagic fever (VHF), Junín virus (JUNV), Machupo virus (MACV), and Bolivian hemorrhagic fever (BHF), while the Old World viruses includes, for example Lujo virus (LUJV), Lymphocytic choriomeningitis virus (LCMV), Morogoro virus (MORV), and LASV. These arenaviruses are both geographically and genetically different (Zhang et al., 2019).
LAFV has two RNA segments in its genome which encode four viral proteins, including zinc-binding protein (Z), RNA polymerase (L), nucleoprotein (NP), and the surface glycoprotein precursor (GP, or spike protein). GP is cleaved into envelope glycoproteins GP1 and GP2 (Cao et al., 1998). They are synthesized as a 76-kDa glycosylated precursor protein (GP-C), which is posttranslationally cleaved into the N-terminal 44-kDa subunit GP1 and the membrane bound 36-kDa subunit Lassa Fever virus GP2. GP1 is responsible for receptor binding (including α-dystroglycan, heparin sulfate, DC-SIGN, etc.) and GP2 mediates membrane fusion interact with each other to form a stable trimer complex on the LASV viral envelope (Li et al., 2016; Hastie et al., 2017). Upon receptor binding, LASV enters the host cell via clathrin- and dynamin-independent endocytosis with subsequent transport to late endosomal compartments, where fusion occurs at low pH (Vela et al., 2007; Rojek et al., 2008).
REFERENCES
- Cao et al. (1998). Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and lassa fever virus. Science 282, 2079–2081.
- Hastie et al. (2017). Structural basis for antibody-mediated neutralization of Lassa virus. Science 356, 923–928. doi: 10.1126/science.aam7260
- Li et al. (2016). Acidic pH-induced conformations and LAMP1 binding of the Lassa Virus glycoprotein spike. PLoS Pathog. 12:e1005418. doi: 10.1371/journal.ppat.1005418
- Racsa et al. (2016). Viral Hemorrhagic Fever Diagnostics. Clin Infect Dis. 62: 214–219.
- Rojek et al. (2008). Different mechanisms of cell entry by human-pathogenic old world and new world arenaviruses. J. Virol. 82, 7677–7687. doi: 10.1128/JVI.00560-08
- Vela et al. (2007). Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 369, 1–11. doi: 10.1016/j.virol.2007.07.014
- Zhang et al. (2019). Crystal Structure of Refolding Fusion Core of Lassa Virus GP2 and Design of Lassa Virus Fusion Inhibitors. Front. Microbiol.