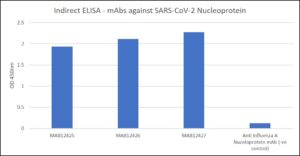
Indirect ELISA: mAbs vs. recombinant SARS-CoV-2 (COVID-19) nucleoprotein.
Assays were run as below:
- Antigen was coated at 2 µg/ml PBS overnight at RT, washed 2X with PBS + 0.3% tween-10 (PBST).
- Wells blocked with 1% normal goat serum in PBS a minimum of 30 minutes.
- Antibodies applied at 1 µg/ml PBST for 30 min at RT, washed 2X with PBST. Note: anti Influenza A Nucleoprotein included as a negative control antibody.
- Goat antibody to mouse IgG+ IgM/HRP conjugate (Jackson Immuno) at 0.5 µg/ml in PBST for 30 minutes at RT. Washed 2X with PBST.
- TMB added for 1 minute.
MOUSE ANTI-SARS CORONAVIRUS NUCLEOPROTEIN ANTIBODY (3864)
Mouse anti SARS Coronavirus nucleoprotein antibody (3864) is specific for the nucleocapsid protein of Severe acute respiratory syndrome Coronavirus (SARS-CoV) and also recognises the NP of SARS-CoV-2 (COVID-19) by ELISA. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
PRODUCT DETAILS – MOUSE ANTI-SARS CORONAVIRUS NUCLEOPROTEIN ANTIBODY (3864)
- Anti SARS coronavirus nucleoprotein monoclonal IgG antibody, clone 3864.
- Purified to >90% purity by protein A chromatography or sequential differential precipitations.
- Suitable for use in ELISA and IFA.
- Anti SARS coronavirus nucleoprotein does not react with human Coronavirus 229E and OC43, feline FIP-1, FIP-2, canine Coronavirus TGEV or mouse hepatitis virus.
BACKGROUND
Severe acute respiratory syndrome (SARS) is a lower respiratory tract illness that was first reported in patients from the Guandong Province of China in November 2002. The causative agent, which was previously unknown, was isolated in 2003 and named as SARS coronavirus (SARS-CoV). The SARS coronavirus is an enveloped, single-stranded, positive RNA virus of the family Coronoviridae (NCBI) The virus is thought to have a zoonotic origin, with the horseshoe bat being the primary natural reservoir, but this has not been confirmed. Mammals, including the palm civet, may act as intermediate hosts.
In 2003, the SARS coronavirus spread rapidly and affected over 8,000 people in 26 countries. The rapid spread of SARS-CoV is thought to be due to person-to-person transmission of the virus via aerosol droplets or close contact with infected individuals. Since the end of the SARS epidemic, cases of SARS have only occurred in laboratory workers that have been accidently infected (WHO). The symptoms of SARS infection are like influenza and include fever, malaise, muscle pain, headache, diarrhoea and shivering. Clinical symptoms may also include coughing and shortness of breath. Respiratory distress may rapidly develop in some patients, resulting in death. SARS disease has a high rate of mortality and resulted in 774 deaths during the first epidemic in 2003. Currently, no licenced vaccine is available for the prevention of SARS infection. SARS continues to be of global health concern due to the rapid spread of the virus, the high mortality rate and the fears of a future SARS outbreak.
REFERENCES