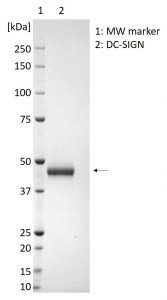
SDS-PAGE showing 1µg purified DC-SIGN protein (indicated by arrow).
Human DC-SIGN, His-Tag
Price range: $798.78 through $2,726.36 excl. VAT
Recombinant human dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) with N-terminal His-tag.
HUMAN DC-SIGN, HIS-TAG
Recombinant human dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) with N-terminal His-tag.
PRODUCT DETAILS – HUMAN DC-SIGN, HIS-TAG
- Recombinant human dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) expressed from HEK293 cells (NCBI Accession Number: NP_066978.1).
- Includes amino acids 59-404 and an N-terminal His-tag.
- Greater than 95% purity by SDS-PAGE and buffered in DPBS, pH7.4.
BACKGROUND
Human DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) also known as CD209 (Cluster of Differentiation 209) is encoded by the CD209 gene (Curtis, et al., 1992). It is a 44-kDa type II integral membrane protein. The protein is organized into three distinct domains: an N-terminal transmembrane domain, a tandem-repeat neck domain and C-type lectin carbohydrate recognition domain. The extracellular region consisting of the C-type lectin and neck domains has a dual function as a pathogen recognition receptor and a cell adhesion receptor by binding carbohydrate ligands on the surface of microbes and endogenous cells. The neck region is important for homo-oligomerization which allows the receptor to bind multivalent ligands with high avidity. Variations in the number of 23 amino acid repeats in the neck domain of this protein are rare but have a significant impact on ligand binding ability.
DC-SIGN is present on the surface of macrophages (and dendritic cells) and recognises and binds to mannose type carbohydrates (high-mannose-containing envelope glycoproteins) which are commonly found on viruses, bacteria and fungi. This binding interaction then activates phagocytosis (McGreal, et al., 2005).
DC-SIGN is a receptor for several viruses including HIV, Hepatitis C, Dengue, Ebola and CMV (Lozach, et al., 2004; Lozach, et al., 2007; Geijtenbeek, et al., 2000) and binding allows them to infect T-cells from dendritic cells. This is an essential process for HIV infection (Wu, et al., 2002) where in the initial stages of dendritic cell infection, the HIV gp120 protein causes co-internalization of DC-SIGN and HIV virions. The dendritic cell then migrates to the cognate lymphoid organ, whereupon recycling of the DC-SIGN/HIV virion complex to the cell periphery facilitates HIV infection of CD4 T cells by interaction between DC-SIGN and ICAM-3 (van den Berg & Geijtenbeek, 2013).
Dengue virus (serotypes 1-4) also use DC-SIGN to infect dendritic cells.THP-1 cells have been shown to become susceptible to Dengue infection after transfection of DC-SIGN (or its homologue L-SIGN). However, infection of dendritic cells is blocked by anti–DC-SIGN antibodies, but not by antibodies to other molecules on these cells. Viruses produced by dendritic cells are subsequently infectious for DC-SIGN (and L-SIGN) bearing THP-1 cells and other permissive cell lines (Tassaneetrithep, et al., 2003).
DC-SIGN (and L-SIGN) also bind Ebola virus glycoproteins and dendritic cells expressing DC-SIGN are more efficiently infected by Ebola (Alvarez, et al., 2002). CMV envelope glycoprotein B is a viral ligand for DC-SIGN (and L-SIGN). Blocking DC-SIGN with antibodies inhibit dendritic cell infection and conversely expression of DC-SIGN (or L-SIGN) rendering cells permissive to CMV infection (Baribaud, et al., 2002). Therefore, DC-SIGN is a promising target for designing therapies to block pathogen infection.
REFERENCES
- Alvarez, C. P. et al., 2002. C-type lectins mediate cellular entry by Ebola virus in cis and in trans. J Virol., 76(13), pp. 6841-4.
- Baribaud, F. et al., 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. J Virol., 76(18), pp. 9135-42.
- Curtis, B. M., Scharnowske, S. & Watson, A. J., 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. U.S.A., 89(17).
- Geijtenbeek, T. B. et al., 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell, 100(5), p. 587–97.
- Lozach, P. Y. et al., 2004. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J. Biol. Chem., 279(31), p. 32035–45.
- Lozach, P. Y., Burleigh, L., Staropoli, I. & Amara, A., 2007. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol. Biol., Volume 379, p. 51–68.
- McGreal, E., Miller, J. & Gordon, S., 2005. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol., 17(1), pp. 18-24.
- Tassaneetrithep, B. et al., 2003. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J Exp Med., 197(7), p. 823–829.
- van den Berg, L. M. & Geijtenbeek, T. B., 2013. Antiviral immune responses by human langerhans cells and dendritic cells in HIV-1 infection. Advances in Experimental Medicine and Biology, Volume 762, p. 45–70.
- Wu, L. et al., 2002. Functional Evaluation of DC-SIGN Monoclonal Antibodies Reveals DC-SIGN Interactions with ICAM-3 Do Not Promote Human Immunodeficiency Virus Type 1 Transmission. J. Virol., 76(12), p. 5905–14.