New tools for HIV diagnosis: Discover our gp140 portfolio!
Our solutions to the HIV assay manufacture challenges
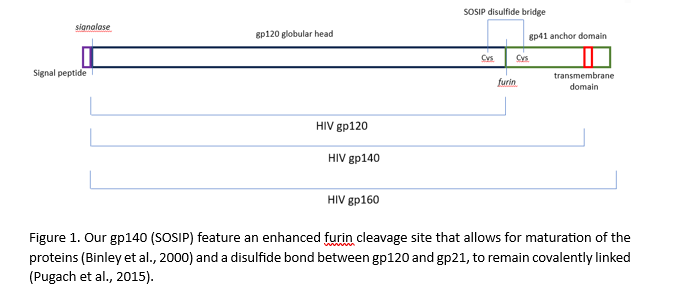
The viral entry receptor for HIV-1 and HIV-2 is the Envelope protein gp160, a transmembrane glycoprotein featuring the mature glycoprotein gp120 and the transmembrane protein gp41. Both neutralising and non-neutralising patient antibodies are targeted against gp160, making this protein an exciting diagnostic and vaccine target. gp160 is synthesised as a polyprotein and cleaved by the host-resident protease furin (Checkley et al., 2011).
Removal of the intravirion and transmembrane domains allows for production of a soluble secreted version of gp160 termed gp140. The Native Antigen Company now offers untagged HIV-1 glycoprotein gp140 group M subtypes A, B, C, CRF01-AE, group O, and HIV-2 glycoprotein gp140, enabling a new generation of diagnostic assays and structural studies.
To learn more about our comprehensive HIV antigen range.
At The Native Antigen Company we also offer a variety of HIV antibodies to further support your R&D

References
Binley, J.M. et al. (2000) ‘A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and GP41 subunits is an antigenic mimic of the trimeric virion-associated structure’, Journal of Virology, 74(2), pp. 627–643. doi:10.1128/jvi.74.2.627-643.2000.
Checkley, M.A., Luttge, B.G. and Freed, E.O. (2011) ‘HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation’, Journal of Molecular Biology, 410(4), pp. 582–608. doi:10.1016/j.jmb.2011.04.042.
Pugach, P. et al. (2015) ‘A native-like sosip.664 trimer based on an HIV-1 subtype B env gene’, Journal of Virology, 89(6), pp. 3380–3395. doi:10.1128/jvi.03473-14.
Get in touch
