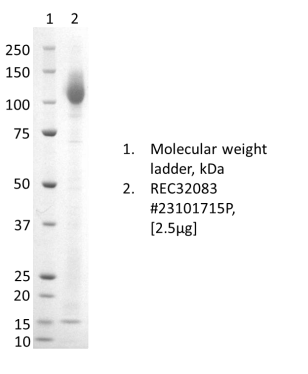
Human immunodeficiency virus subtype A, gp140 (SOSIP), untagged
PRODUCT INFORMATION – Human immunodeficiency virus subtype A, gp140 (SOSIP)
- Produced in HEK293 cells and purified from culture supernatant by lectin affinity chromatography
- Strain KNH1144, NCBI accession number AFJ93242.1
- Untagged (please contact our team to enquire about the conjugation possibilities)
- Suitable for use in ELISA, western blot and lateral flow immunoassay.
BACKGROUND
Human immunodeficiency virus (HIV) is a retrovirus (genus Lentivirus) with a single-stranded, positive-sense RNA genome. Upon entry of the target cell, the viral RNA genome is converted to double-stranded DNA by a virally encoded reverse transcriptase that is present in the virus particle. This viral DNA is then integrated into the cellular DNA by a virally encoded integrase allowing the genome to be transcribed. Once the virus has infected the cell, two pathways are possible: either the virus becomes latent and the infected cell continues to function, or the virus becomes active and replicates, and a large number of virus particles are liberated to infect other cells. Infection with HIV leads to a condition in which the immune system begins to fail, leading to opportunistic infections. HIV primarily infects cells in the human immune system including CD4 T cells, macrophages and dendritic cells. Infection subsequently results in low levels of CD4 T cells via direct viral killing of infected cells, increased rates of apoptosis in infected cells and killing of infected CD4 T cells by CD8 cytotoxic lymphocytes that recognize infected cells. When CD4 T cell numbers decline below a critical level, cell-mediated immunity is lost, and the body becomes progressively more susceptible to opportunistic infections.
Gp41 also known as glycoprotein 41 is a subunit of the envelope protein complex of human immunodeficiency virus (HIV). It is a transmembrane protein that contains several sites within its ectodomain that are required for infection of host cells and as a result it has received much attention as a potential target for HIV vaccines. Essentially, gp41 mediates fusion between viral and cellular membranes (Chan et al., 1997). Gp41 is coded with gp120 as one gp160 by the env gene of HIV. Gp160 is then extensively glycosylated and proteolytically cleaved by a host cellular protease, furin. The high glycosylation of the env coded glycoproteins allows them to escape the human body’s immune system. Gp41 is less glycosylated that gp120 and more conserved (less prone to genetic variations). Once gp160 has been cleaved into its individual subunits, the subunits are then associated non-covalently on the surface of the viral envelope.
When they are non-covalently bound to each other Gp41 and gp120 form the envelope spike complex, composed of a heterotrimer of three gp41 and three gp120. The complex is responsible for the attachment, fusion, and ultimately the infection of host cells. Gp120 sits on the surface of the viral envelope and gp41 is the transmembrane portion of the spike complex with part of the glycoprotein buried within the viral envelope. The membrane-proximal external region (MPER) is a highly conserved region of gp41 subunit near the viral envelope surface, and plays a key role in membrane fusion. It is seen as an attractive vaccine target using broadly neutralizing antibodies (bNAbs) or non-neutralizing, yet protective, antibodies (Liu et al., 2018).
Natural noncovalent interactions between the six subunits of the HIV-1 envelope glycoprotein complex cannot maintain the trimeric structure of recombinantly expressed proteins. TNAC customers reported that the recombinant gp41 antigen alone is typically insoluble and difficult to handle without buffers containing high concentrations of ionic detergents, making it poorly suited to IVD assay manufacturing. A disulfide bond is introduced between gp120 and gp41 to stabilise the native-like structure of the HIV-1 glycoprotein complex, providing a bridge between gp120 and the gp41 soluble domain, gp21. The resulting gp140 antigens (SOSIP) mimic the antigenic properties of the native HIV envelope proteins, which, as the main target of the neutralising antibody responses, are critical in assay development and vaccine design studies (Pugach et al., 2015). The recombinant gp140 protein is soluble and stable in simple physiological buffers for convenient assay development and production (Harris et al., 2011). Through altering the amino acid sequence of the native furin cleavage site, we promoted enhanced cleavage of the gp140 polyprotein, resulting in optimal presentation of the gp120:gp21 ectodomain antigenic target as described in Binley et al., 2000.
REFERENCES
- Binley, J.M. et al. (2000) ‘A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and GP41 subunits is an antigenic mimic of the trimeric virion-associated structure’, Journal of Virology, 74(2), pp. 627–643. doi:10.1128/jvi.74.2.627-643.2000.
- Chan et al. (1997). Core Structure of gp41 from the HIV Envelope Glycoprotein. Cell. Volume 89, Issue 2, 18 April 1997, Pages 263-273.
-
Harris, A. et al. (2011) ‘Trimeric HIV-1 glycoprotein GP140 immunogens and native HIV-1 envelope glycoproteins display the same closed and open quaternary molecular architectures’, Proceedings of the National Academy of Sciences, 108(28), pp. 11440–11445.
- Liu et al. (2018). The development of HIV vaccines targeting gp41 membrane-proximal external region (MPER): challenges and prospects. Protein Cell. 9(7): 596–615.
-
Pugach, P. et al. (2015) ‘A native-like sosip.664 trimer based on an HIV-1 subtype B env gene’, Journal of Virology, 89(6), pp. 3380–3395.

![Human Immunodeficiency Virus GP120 Protein [HIV-1/Clade B (89BZ_167)]](https://thenativeantigencompany.com/wp-content/uploads/1970/01/Misc-Virus-33-M-1.jpg)