The field of diagnostics is rapidly developing, yet ELISA and PCR methods remain the most commonly used techniques in the diagnosis of bacterial and viral infections. In this blog, we discuss the advantages of using serological methods over molecular, PCR-based methods, the different ELISA formats that are most commonly used, and introduce our newly-extended range of highly-specific ELISAs for infectious disease research.
PCR or ELISA?
Historically, the diagnosis of infection was based solely on the clinical presentations of a disease. However, with the emergence of co-circulating diseases that share similar clinical manifestations, more and more rigorous diagnostic tests are required to diagnose infections with fidelity. Accurate diagnosis is crucial, not only in providing an appropriate treatment, but also in the rapid response to outbreaks before they can develop and proliferate. The two most common types of in vitro diagnostic methods for detecting pathogens are molecular (PCR-based) or serological (immunoassay-based).
Polymerase Chain Reaction (PCR) methods directly detect the pathogen by its genetic material, amplifying small traces of the pathogen’s DNA or RNA by a cyclic amplification process. PCR is valuable in the very early stages of infection when the body hasn’t yet produced antibodies against the pathogen. However, the ability of PCR to detect a pathogen’s genetic material early in an infection is also its downfall. Shortly after the pathogen has been cleared from the immune system, or has become latent (viruses), genetic material is no longer available for detection in the bloodstream. This leaves only a short window during the acute stage of infection and does not allow retrospective analysis of historic infections.
Enzyme-Linked ImmunoSorbent Assays (ELISAs) are a type of immunoassay used to detect the presence of antigens or antibodies in blood sera. These assays take on various different formats, but essentially consist of an antigen or antibody that is immobilised on a surface, which is then bound by a specific enzyme-linked antibody to produce a detectable signal. Unlike genetic material, antibodies raised against a pathogen will persist in the bloodstream for many years after infection and also vary in type and concentration, according to the stage of infection, or time after exposure to the pathogen. This makes ELISAs invaluable for studying the epidemiology of disease over many years and can be used to study present and past disease prevalence in different populations over time. When looking at the practical aspects of diagnosis, PCR requires specialist lab equipment and training, while serological methods are simple to carry-out and require only basic equipment. ELISA’s cousin, the Lateral Flow Assay (LFA), is an even more compact and robust immunoassay that is becoming widely adopted in point of care (PoC) diagnostic tests.
Lets look at some of the different ELISA formats that are used in infectious disease:
ELISA formats
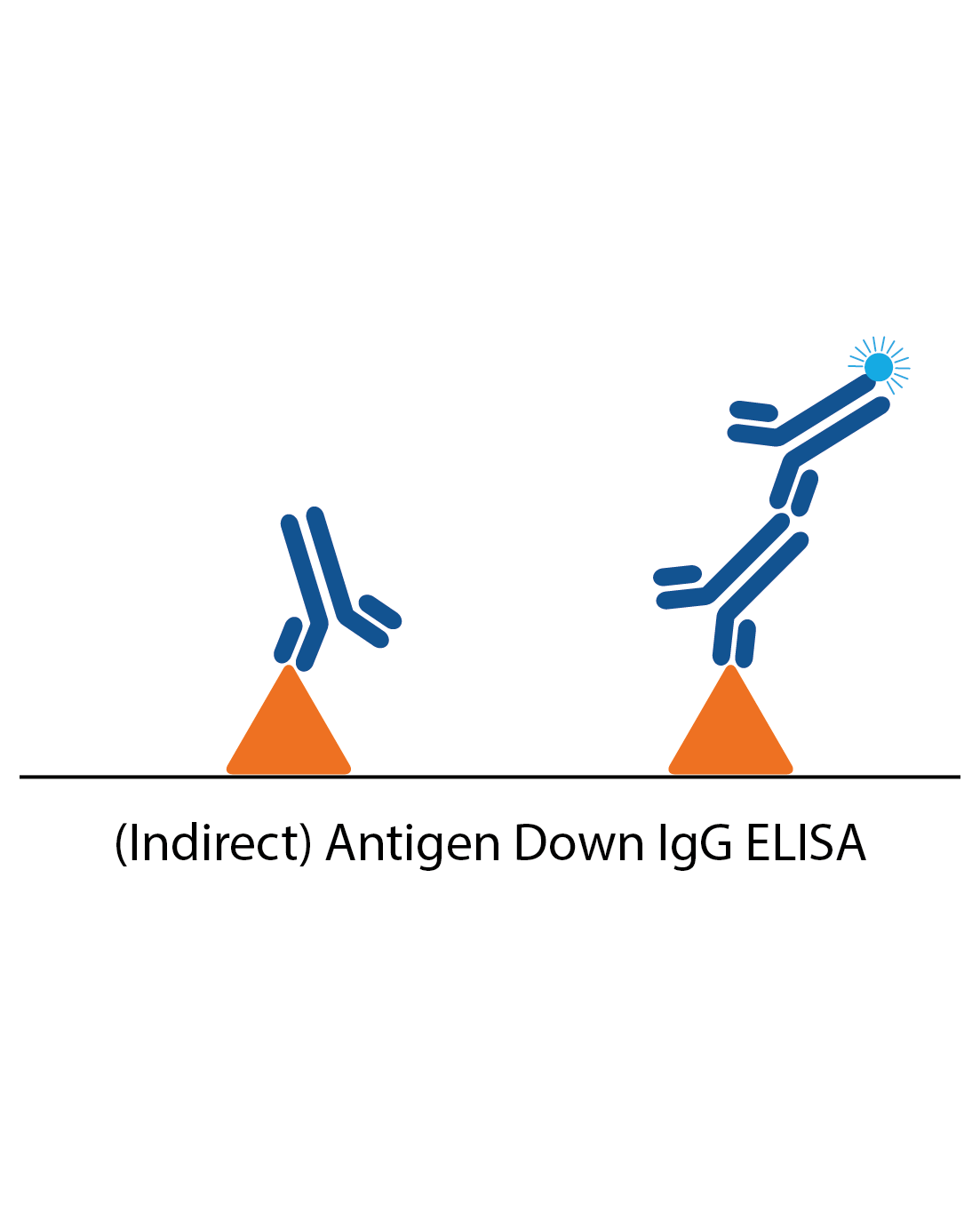
IgG antigen down ELISA
IgG antigen down ELISAs detect IgG antibody responses to a particular antigen and are one of the simplest ELISAs. The plate is coated with antigen and the patient sample applied. Specific IgG in the sample will then bind and be detected by anti-human IgG antibodies to produce a signal.
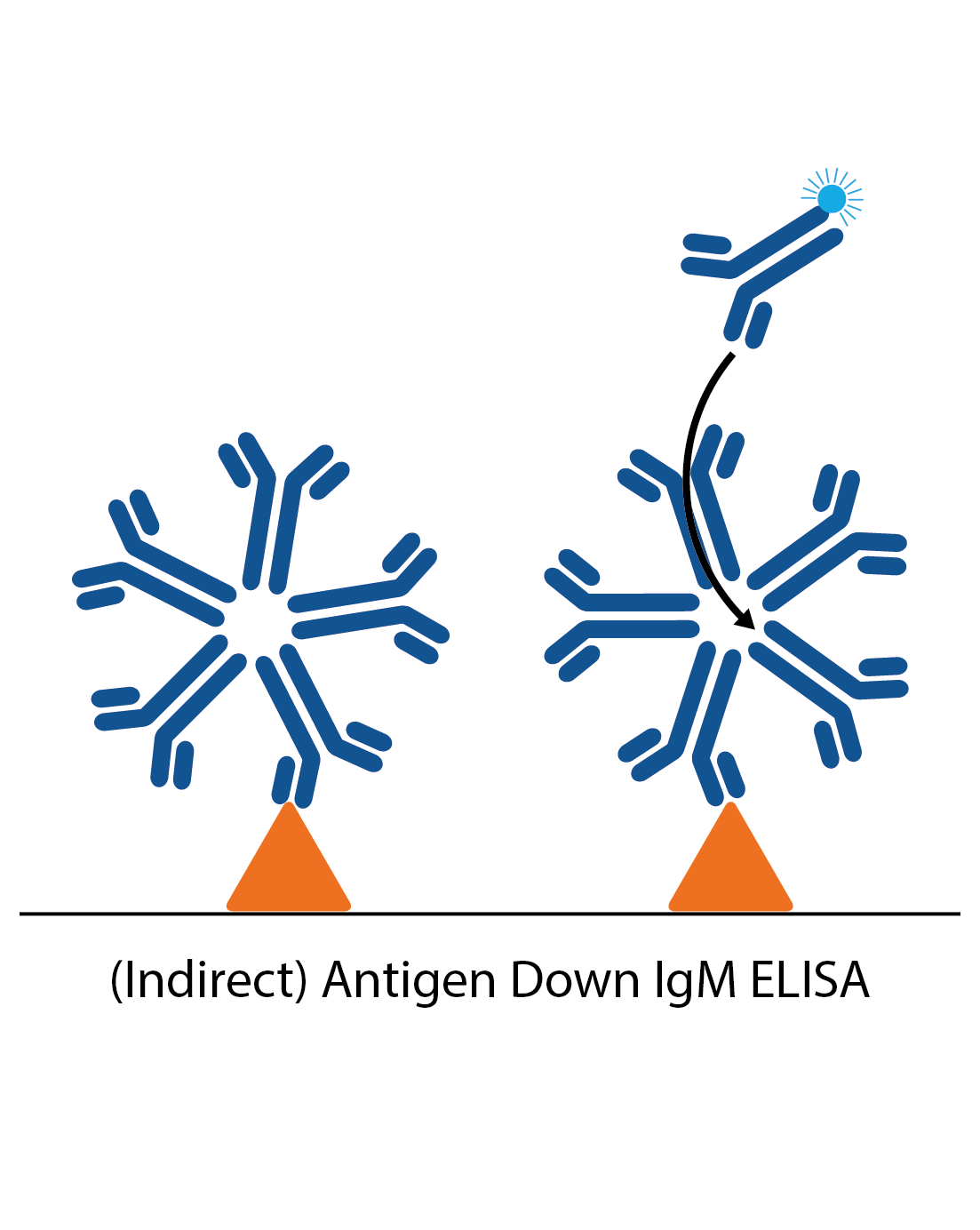
IgM antigen down ELISA
IgM antigen down ELISAs detect IgM antibody responses to a particular antigen. The plate is first coated with antigen and the patient sample is applied. Specific IgM in the sample then binds and is detected by an anti-human IgM antibody. In many cases reactive IgG in the sample needs to be removed, which can be done by using an “IgG stripper” that is added to the sample during the ELISA process (IgG needs to be removed as it is present in much higher amounts than IgM, and will usually be of higher affinity. It can therefore block IgM binding).
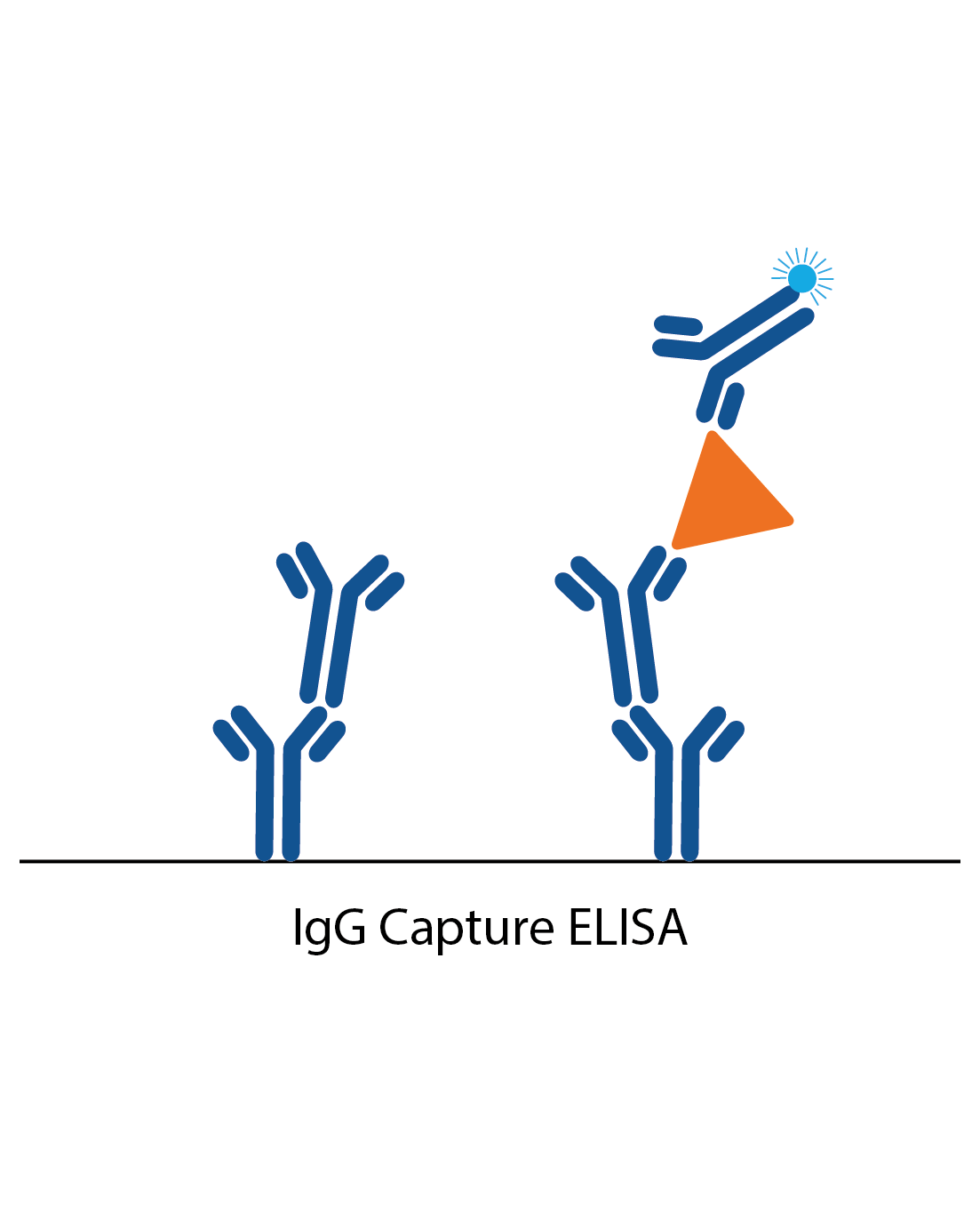
IgG capture assay
In an IgG capture assay, the ELISA plate is coated with anti-human IgG. When the patient sample is added, all the IgG antibodies in the sample bind to these. Next, a specific antigen is added, which is ‘captured’ by the patient IgG if there is specific IgG antibody to it from the patient sample. Binding of the antigen is then detected with a specific antibody labelled to an enzyme (or antigen can be directly labelled in some cases).
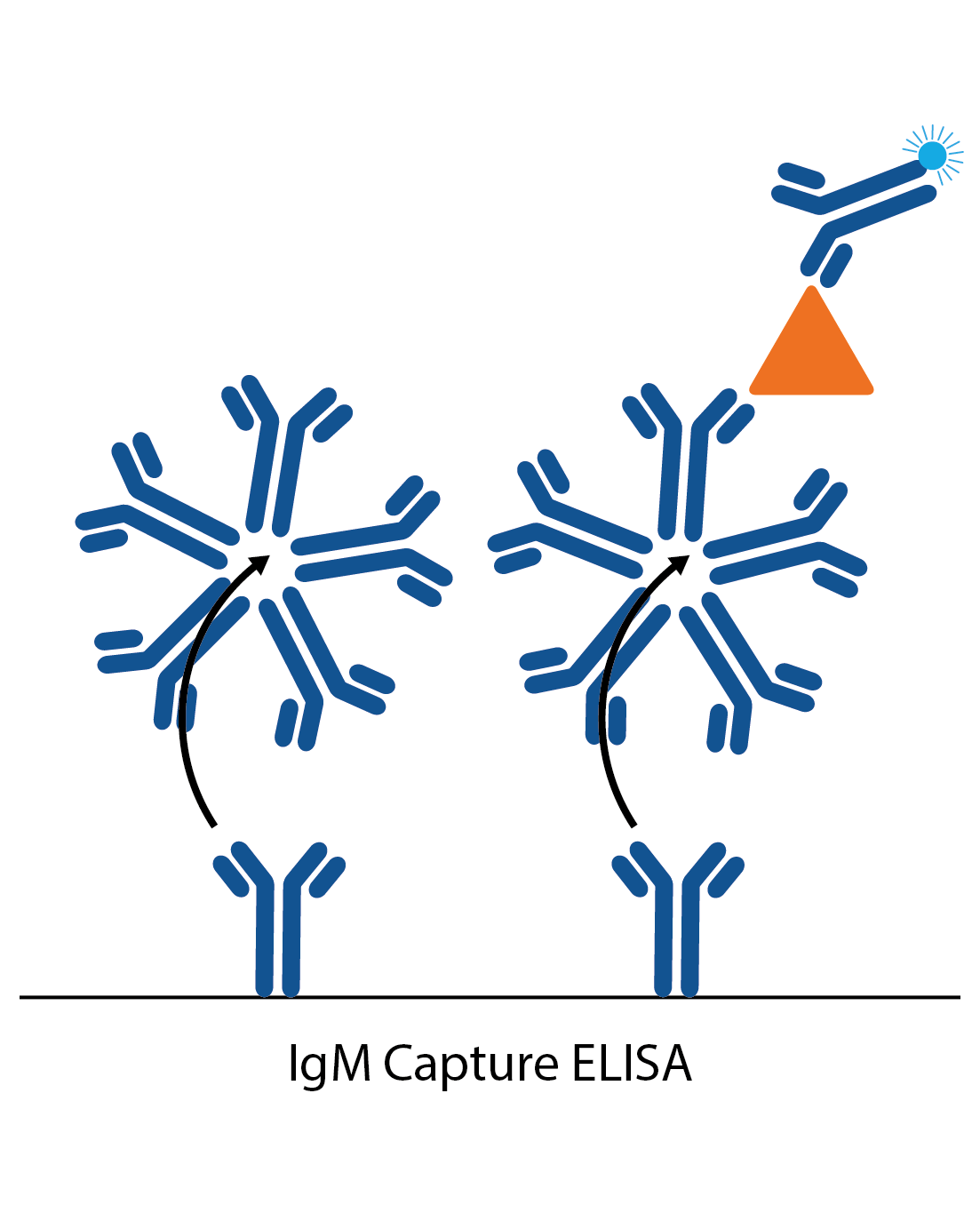
IgM capture assay
IgM capture assays are much the same as the IgG captures, but are more commonly used as they avoid the need for IgG stripping. The ELISA plate is coated with anti-human IgG. When the patient sample is added, all the IgM antibodies in the sample bind to these. Next, a specific antigen is added, which is ‘captured’ by the well-bound IgG if there is specific IgM antibody to it from the patient sample. Binding of the antigen is then detected with a specific antibody labelled to an enzyme (or antigen can be directly labelled in some cases).
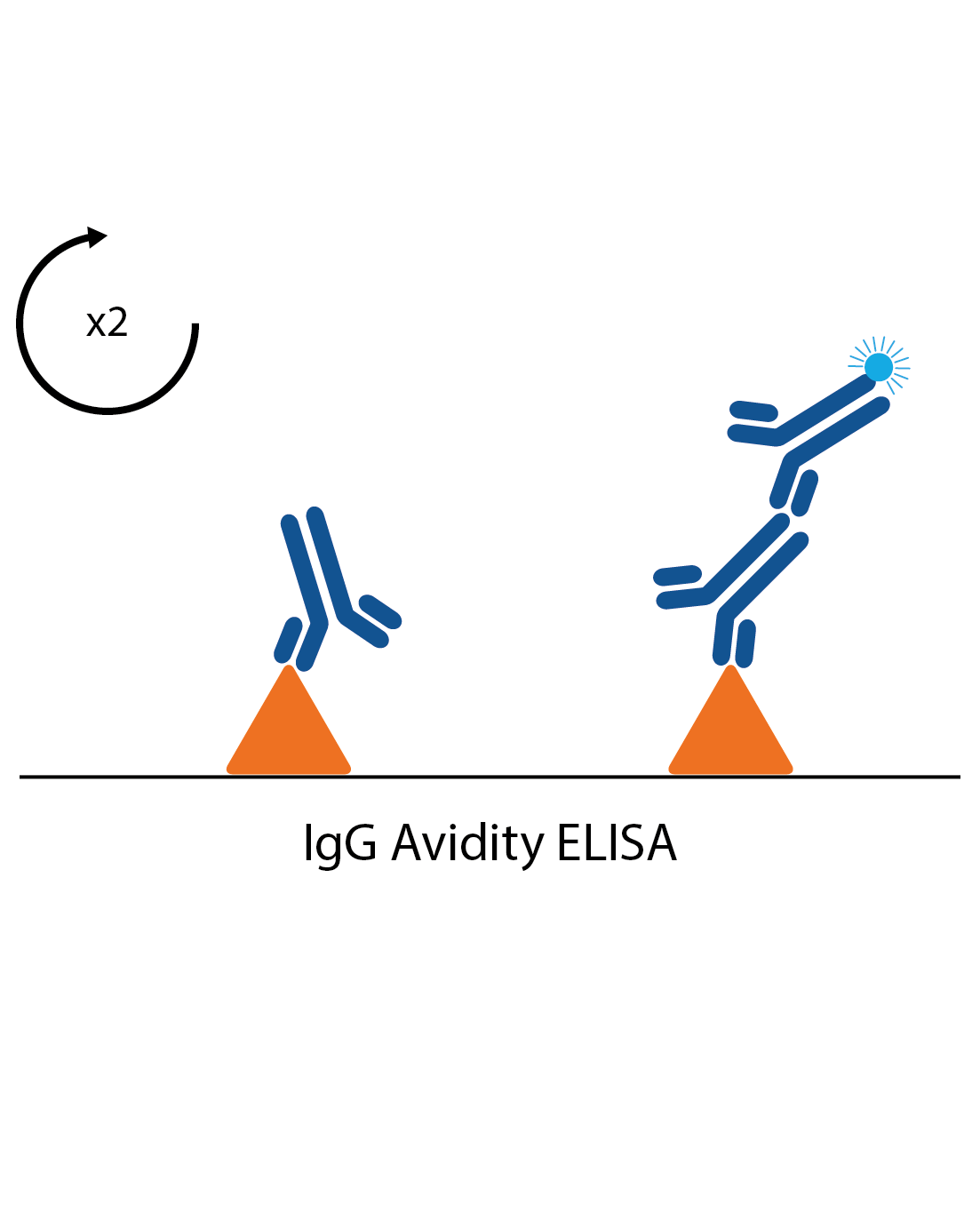
IgG avidity assays
In some cases measuring the strength of an antibody response can give an indication if an infection is a first infection or second exposure. These are often used in cases such as CMV and Rubella infection, where it is important to know if any exposure during pregnancy is primary or secondary. Avidity assays are standard IgG assays, but with all samples tested twice. One of the pairs is then treated with a reagent to remove low affinity antibodies. If most of the antibodies are shown to be low affinity, the infection is likely to be a primary infection.
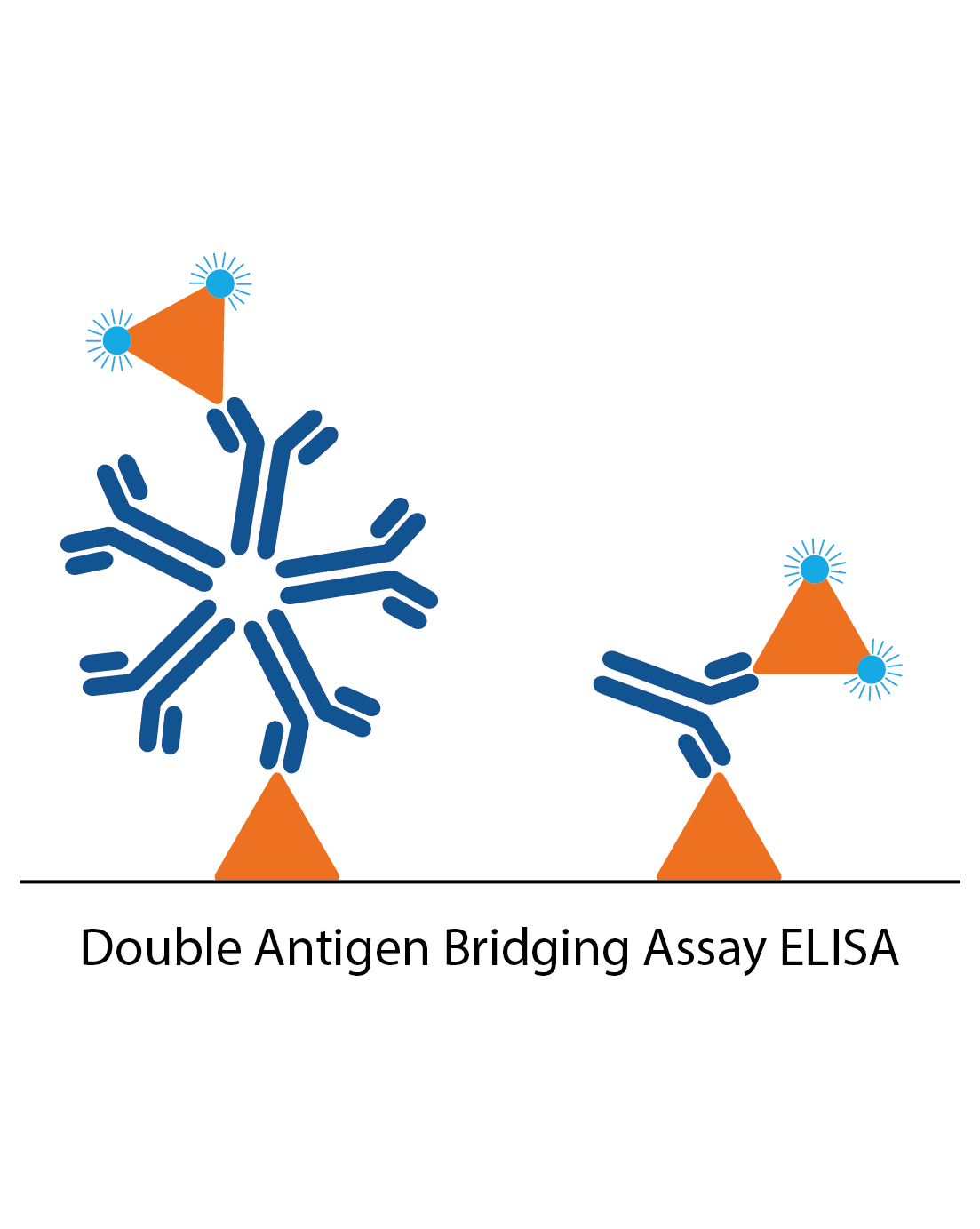
DABA assays
Unlike conventional ELISA formats, double antigen bridging assays (DABA) use an antigen sandwich, with the second part of the sandwich being an antigen conjugated to an enzyme to visualise specific detection. DABAs are total antibody assays, meaning that they detect all immunoglobulin types and classes. This immunoassay design poses several advantages to conventional ELISA formats, especially when trying to reduce the effects of cross-reactive antibodies.
Detection methods
While assay design can vary considerably, the final stage of any ELISA is ultimately the detection step. Unless a radioactive probe is used, this most commonly involves the use of an enzyme substrate that is converted to a detectable product. A Broad range of enzyme-conjugated antibodies can be made to detect a signal by different imaging methods, such as light, colour, fluorescence or luminescence. Choosing your detection method is therefore a trade-off between, practicality, time and sensitivity. For example, an IgG antigen down ELISA might use an anti-human IgG antibody conjugated to either HRP or Alkaline Phosphatase enzymes in the simplest format. Alternatively, you could conjugate the detector antibody to Biotin and then add a further layer of Streptavidin:HRP or Alkaline Phosphatase to provide extra sensitivity, but will do so at the expense of increased assay time (which may be less practical in high-throughput or rapid response applications).
Our new range of ELISAs
Following the success of our Zika ELISAs released last year, we are expanding our immunoassay range to include kits for various other viral and bacterial diseases. These research-use-only ELISAs are highly specific and can be used for a range of research applications, including epidemiological studies. Our highly experienced assay development team are continuously developing new assays, alongside providing custom and contract services to academia and industry.
Click on previews below to find out about the design and performance of our new ELISA kits:
SARS-CoV-2 Neutralization Assay Development Kit (Brazilian Variant RBD-ACE2)
$3,939.23 excl. VAT
SARS-CoV-2 Neutralization Assay Development Kit (Delta Variant RBD-ACE2)
$3,939.23 excl. VAT
SARS-CoV-2 Neutralization Assay Development Kit (Kerala Variant RBD-ACE2)
$3,939.23 excl. VAT
SARS-CoV-2 Neutralization Assay Development Kit (Lambda Variant RBD-ACE2)
$3,939.23 excl. VAT
SARS-CoV-2 Neutralization Assay Development Kit (South African Variant RBD-ACE2)
$3,939.23 excl. VAT
SARS-CoV-2 Neutralization Assay Development Kit (United Kingdom Variant RBD-ACE2)
$3,939.23 excl. VAT
Zika Virus Total Antibody Detection Assay Development Kit
$771.90 excl. VAT

