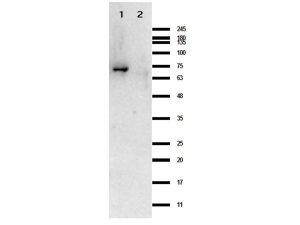
BORRELIA BURGDORFERI SENSU STRICTO (B31) CRASP-1 PROTEIN
Recombinant Borrelia burgdorferi CRASP-1 protein, fused to an MBP-tag and produced in E. coli (>90% purity).
PRODUCT DETAILS – BORRELIA BURGDORFERI SENSU STRICTO (B31) CRASP-1 PROTEIN
- Recombinant Borrelia burgdorferi CRASP-1 protein (NCBI Accession number: NP_045741).
- Greater than 90% pure (by SDS-PAGE) in 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 and 0.01% (w/v)
Sodium Azide.
BACKGROUND
Complement Regulator-Acquiring Surface Protein 1 (CRASP-1, CspA) is a 25.9 kDa multifunctional protein, encoded by the spirochete B. burgdorferi, which is carried by Ixodes ticks. Strain B31 is the type strain (ATCC 35210) for this organism and was derived by limited dilutional cloning from the original Lyme-disease tick isolate obtained by A. Barbour (Johnson, et al., 1984). Native CRASP-1 represents a homodimer with a previously unknown complex protein fold (Cordes, et al., 2005).
CRASP-1 and four additional immune evasion proteins bind combinations of human plasma regulators, including collagen I, collagen III, collagen IV, fibronectin, laminin, factor H-like protein 1 (FHL-1) (resulting in inhibition of complement activation in mammals) and Human Bone Morphogenic Protein 2. These interactions may contribute to adhesion, bacterial colonization, and organ tropism to promote dissemination of B. burgdorferi in the host.
Borrelia species contain a large number of both linear and circular plasmids, some of which appear to be repeated or contain fragments of other genes. These regions may serve as pools of variability for the survival of Borrelia in its multiple environments during its life cycle. Multiple copies of sequences analogous to CRASP-1 genes have been detected in Borrelia plasmids. In addition, the sequence for CRASP-1 contains a repeated sequence folded into a stable stem loop structure, typical of RNA genes (Kraiczy, et al., 2004).
CRASP-1 also binds the human terminal complement components C7 and C9 and blocks assembly and membrane insertion of the terminal complement complex (TCC), as confirmed by haemolytic assays, inhibiting polymerization of C9. When CRASP-1 was expressed ectopically on the surface of serum-sensitive B. garinii, it blocked TCC assembly and induced serum resistance in the transformed bacteria. By inducing serum resistance and terminal complement pathway inhibition, B. burgdorferi is able to survive in the hostile environment of human plasma (Hallström, et al., 2010).
REFERENCES
- Cordes, F. S. et al., 2005. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol. , Volume 3, pp. 276-7.
- Hallström, T. et al., 2010. • Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis., 202(3), pp. 490-8.
- Johnson, R.C., et al. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol, 34, pp. 496–497.
- Kraiczy, P. et al., 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. , 279(4), pp. 2421-9.