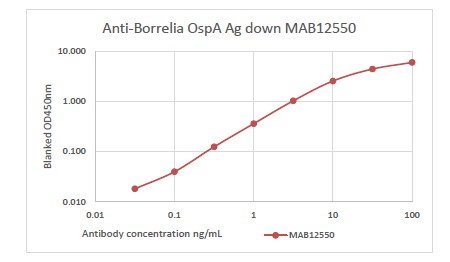
Human Anti-Borrelia burgdorferi OspA IgM, Recombinant
Price range: $674.73 through $2,867.60 excl. VAT
Anti-Borrelia burgdorferi OspA recombinant human IgM, purified by ion exchange chromatography and dialysis.
Human Anti-Borrelia burgdorferi OspA IgM, Recombinant
Anti-Borrelia burgdorferi OspA recombinant human IgM, purified by ion exchange chromatography and dialysis.
PRODUCT DETAILS – Human Anti-Borrelia burgdorferi OspA IgM
- Isotype: human IgM
- Clone number: M1369
- Presented as Liquid in DPBS Buffer.
BACKGROUND
Lyme disease, caused by the spirochete Borrelia burgdorferi and related species, is the most common vector-borne illness in the Northern Hemisphere. If untreated, it can progress from early skin manifestations to joint, cardiac, and neurological complications. Accurate serological testing for Borrelia-specific IgG and IgM antibodies remains essential for timely diagnosis, infection staging, and effective patient management in endemic regions. The performance of diagnostic platforms such as ELISA and Western blot tests relies heavily on the quality and specificity of the antibody reagents employed (CDC, 2023).
Recombinant anti-Borrelia OspA antibodies specifically recognize the Outer Surface Protein A (OspA), a key Borrelia lipoprotein expressed in the tick midgut. Compared with traditional antibodies derived from infected sera, recombinant OspA antibodies offer defined specificity, high batch-to-batch consistency, and superior purity—critical factors for diagnostic assay reliability and reproducibility (Busby et al., 2018; Dunn et al., 2015).
In clinical applications, recombinant anti-Borrelia OspA IgG antibodies serve as essential controls, calibrators, and reagents for serological Lyme disease assays. Their well-characterized nature helps overcome the challenges of variable antibody responses and inconsistent interpretation across laboratories. By incorporating recombinant antigens like OspA, diagnostic developers can improve assay sensitivity and accuracy, validate diagnostic performance, and strengthen global Lyme disease surveillance programs (Wilske et al., 2007; Steere et al., 2016).
REFERENCES
CDC. (2023). Lyme Disease. Centers for Disease Control and Prevention.
Busby, B. et al. (2018). Serodiagnosis of Lyme disease with recombinant antigens. Clinical Microbiology Reviews, 31(1), e00057-17.
Dunn, J. J. et al. (2015). Recombinant antigens for the serodiagnosis of Lyme disease. Journal of Clinical Microbiology, 53(1), 10–19.
Steere, A. C. et al. (2016). The serodiagnosis of Lyme disease. Current Problems in Dermatology, 48, 118–127.
Wilske, B. et al. (2007). Performance of Borrelia burgdorferi sensu lato immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) using different recombinant antigens. Journal of Clinical Microbiology, 45(9), 2974–2980.
You may also like…
Rabbit Anti-Borrelia burgdorferi sensu stricto (B31) OspA Antibody
Price range: $220.72 through $1,121.09 excl. VAT
Rabbit Anti-Borrelia burgdorferi sensu stricto (B31) OspC Antibody
Price range: $220.72 through $1,121.09 excl. VAT
Borrelia burgdorferi OspA Protein, His-Tag (E. coli)
Price range: $737.83 through $2,518.25 excl. VAT