Human anti-Hepatitis B virus Core HBcAg/HBeAg IgM (clone M1428), Recombinant
Price range: $674.73 through $2,867.60 excl. VAT
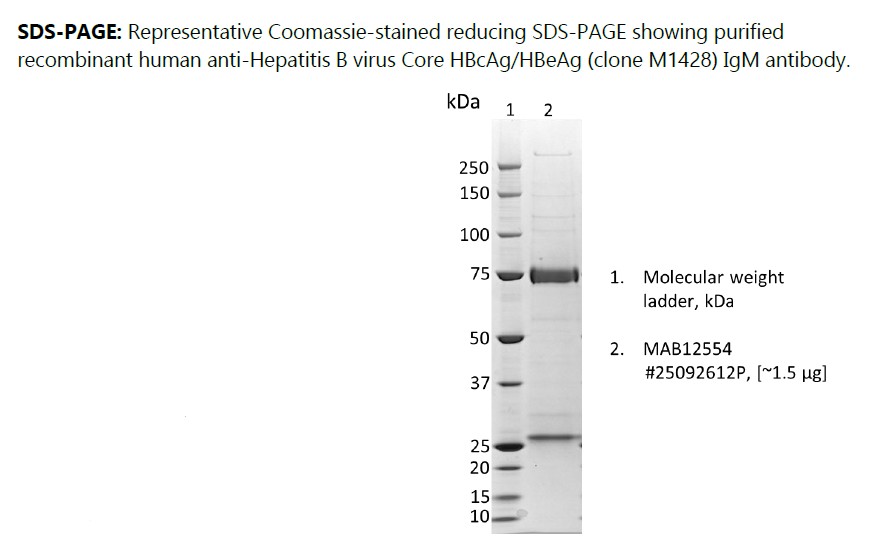
Recombinant human anti-Hepatitis B virus Core HBcAg/HBeAg (clone M1428) IgM antibody, produced in HEK293 cells and purified by ion exchange chromatography, ammonium sulphate precipitation and dialysis.
Human anti-Hepatitis B virus Core HBcAg/HBeAg IgM (clone M1428), Recombinant
Recombinant human anti-Hepatitis B virus Core HBcAg/HBeAg (clone M1428) IgM antibody, purified by ion exchange chromatography and dialysis.
PRODUCT DETAILS – Hepatitis B virus Core HBcAg/HBeAg IgM (clone M1428)
- Isotype: human IgM
- Clone Number: M1428
- Presented as Liquid in 50 mM HEPES, 120 mM NaCl, pH 8.0
BACKGROUND
Hepatitis B virus (HBV) causes acute and chronic liver infections, potentially leading to cirrhosis, liver failure, and liver cancer (CDC, 2023; WHO, 2023). Accurate diagnosis relies on detecting specific viral antigens and antibodies, mainly hepatitis B surface antigen (HBsAg), hepatitis B core antibodies (anti-HBc), and hepatitis B e antigen (HBeAg) or its antibody (anti-HBe), which indicate viral presence, infection phase, and infectivity (CDC, 2023; HepB Foundation, 2018).
Recombinant antibodies targeting key HBV proteins, such as HBcAg and HBeAg, are produced using recombinant DNA technology, providing highly specific, consistent, and pure reagents crucial for diagnostic assays, especially ELISAs. These recombinant antibodies outperform traditional polyclonal antibodies in reliability and reproducibility (Wang et al., 2011; Wei et al., 2013).
Clinically, anti-HBc IgM identifies acute infection, while total anti-HBc (IgG and IgM) indicates prior or ongoing exposure. HBeAg presence marks active viral replication and high infectivity, and anti-HBe emergence signals reduced replication, guiding disease monitoring and treatment (CDC, 2023; Lai et al., 2019).
This focused antibody detection via recombinant reagents enhances diagnostic accuracy, improves clinical management, and supports public health efforts against HBV.
REFERENCES
CDC. (2023). Hepatitis B Information. Centers for Disease Control and Prevention.
World Health Organization. (2023). Hepatitis B: Fact sheet.
Wang, R. Y. et al. (2011). Preparation and characterization of monoclonal antibody against HBcAg. Hybridoma (Larchmt), 30(5), 451-457.
Wei, C. et al. (2013). Development of an indirect ELISA using recombinant HBeAg for detection of anti-HBe antibody. Journal of Virological Methods, 187(2), 241-246.
Lai, C. L. et al. (2019). Hepatitis B e antigen seroconversion: definition, relevance, and implications for clinical practice. Journal of Viral Hepatitis, 26(10), 1162-1170.
You may also like…
Human anti-Hepatitis B virus Core HBcAg/HBeAg IgG (clone M1424), Recombinant
Price range: $346.02 through $1,470.57 excl. VAT
Hepatitis B Virus Core Antigen (HBcAg)
Price range: $752.53 through $2,257.60 excl. VAT
Human anti-Hepatitis B virus Core HBcAg/HBeAg IgM (clone M1426), Recombinant
Price range: $674.73 through $2,867.60 excl. VAT