Human anti-Measles virus IgM (clone M1391), Recombinant
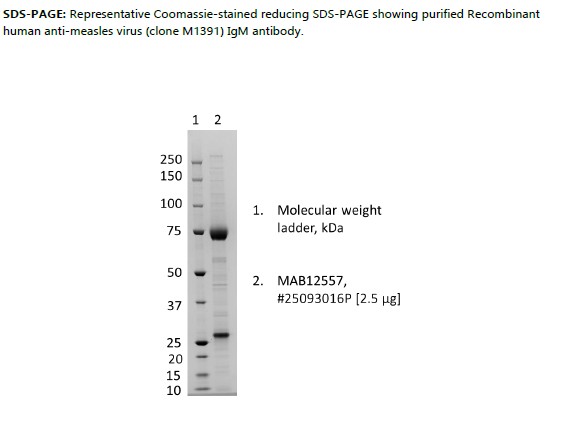
Recombinant human anti-measles virus (clone M1391) IgM antibody, purified by ion exchange chromatography and dialysis.
PRODUCT DETAILS – Human anti-measles virus IgM (clone M1391), Recombinant
- Isotype: human IgM
- Clone Number: M1391
- Presented as Liquid in 50 mM HEPES, 120 mM NaCl, pH 8.5.
Background
Measles is a highly contagious and severe viral infection. Public health authorities, including the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), consistently report that it poses a significant risk of serious complications such as pneumonia and encephalitis, particularly in vulnerable groups such as young children, pregnant women, and immunocompromised individuals. Given this severity, accurate IgG serology testing is essential for confirming immunity, monitoring population protection, and informing public health strategies.
Recombinant anti-measles antibodies typically target key surface glycoproteins of the measles virus—hemagglutinin (H) and fusion (F) proteins—which are critical for viral entry and infection. Research has highlighted the diagnostic value of recombinant proteins in measles testing. Early studies by Bouche et al. (1998) and de Swart et al. (1998) demonstrated that recombinant hemagglutinin protein used in ELISA assays for measles IgG detection shows a strong correlation with traditional neutralization and hemagglutination inhibition tests, offering superior diagnostic sensitivity and specificity.
The use of recombinant technology delivers significant advantages for measles virus diagnostics. Recombinant antibodies offer defined specificity, improved batch-to-batch consistency, and high purity compared with traditional antibody sources, thereby enhancing overall assay reliability (Hust et al., 2019). This reproducibility is particularly important for large-scale immunity surveillance programs.
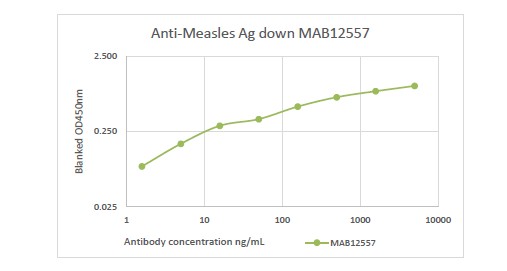
In diagnostic applications, recombinant anti-measles IgG antibodies serve as critical reagents, controls, or calibrators in ELISA platforms for immunity screening and vaccine evaluation. To ensure accurate results and effective public health monitoring, maintaining parallelism and dilution linearity between recombinant calibrators and native antibodies is essential—especially given ongoing challenges with assay variability, even after calibration to international standards (Vandermeulen et al., 2011).
REFERENCES
Bouche, F. et al. (1998). Immunosorbent Assay Based on Recombinant Measles Virus Hemagglutinin Protein. Journal of Virological Methods.
de Swart, R. L. et al. (1998). Measles Virus Fusion Protein- and Hemagglutinin-Transfected Cell Lines as a Sensitive Tool for Detection of Specific Antibodies. Journal of Virological Methods.
Hust, M. et al. (2019). Recombinant Antibodies in Diagnostics: Applications and Challenges. New Biotechnology.
Vandermeulen, C. et al. (2011). Measles Antibody Assays: A Review of the Literature. Clinical and Vaccine Immunology.