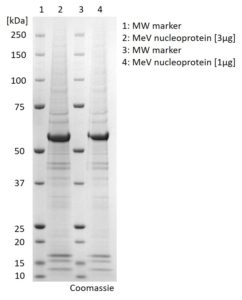
SDS-PAGE: Coomassie-stained SDS-PAGE showing purified Measles virus Nucleoprotein.
Measles Virus Nucleoprotein (HEK293)
$705.96 – $2,474.85 excl. VAT
Measles virus (Rubeola) recombinant nucleoprotein antigen is manufactured in mammalian HEK293 cells for use in diagnostics research and vaccine development.
MEASLES VIRUS NUCLEOPROTEIN (HEK293)
Measles virus (Rubeola) recombinant nucleoprotein antigen is manufactured in mammalian HEK293 cells for use in diagnostics research and vaccine development.
PRODUCT DETAILS – MEASLES VIRUS NUCLEOPROTEIN (HEK293)
- Measles virus nucleoprotein (NCBI accession number P04851.1, AA1-523)
- Recombinant protein produced in HEK293 cells and purified by fractionation of transiently transfected cells.
- Presented in Dulbecco’s phosphate buffered saline (DPBS) pH 7.4. Contains traces of SDS.
BACKGROUND
Measles virus (MV) is the type species of the Morbillivirus genus, which is one of the seven genera of the family Paramyxoviridae. It is a highly contagious, re-emerging, major human pathogen. It is a globally widespread disease that affects children but can also cause disease in unvaccinated adults. Humans are the only known host of measles virus and infection is spread from person-to-person via respiratory aerosol droplets, nasal secretions or through direct contact with infected individuals.
MVs are enveloped and contain single-stranded, negative-sense RNA genomes of 9-19 kbp. The viral RNA is bound by thousands of copies of the viral nucleoprotein, forming the helical nucleocapsid (NC). The MV single-stranded RNA genome is enclosed by nucleocapsid proteins into helical ribonucleoprotein (RNP) complexes comprising of nucleo- (N), phospho- (P), and large (L) proteins. Encapsidated RNAs act as templates for transcription and replication by the viral RNA-dependent RNA-polymerases (RdRPs) complexes (Gutsche et al., 2015). Unencapsidated viral genomic RNA is transcriptionally inactive and not infectious (Duprex et al., 2002). N protein comprises two distinct parts: Folded Ncore and Ntail, an intrinsically disordered domain, containing a linear motif with α-helical propensity (residues 484–502) (Guseva et al., 2019).
MeV has an RNA-dependent RNA polymerase composed of two viral polypeptides, a large protein, L, and a phosphoprotein, P, for transcription and replication of their genome. The L protein confers the RNA polymerase activity on the complex while the P protein acts as a transcription factor. Replication requires a viral RNA-dependent RNA polymerase (RdRP) consisting of the large (L) polymerase protein complexed with the homo-tetrameric phosphoprotein (P) (Du Pont et al., 2019).
REFERENCES
- Du Pont et al. (2019). Bipartite interface of the measles virus phosphoprotein X domain with the large polymerase protein regulates viral polymerase dynamics. PLoS Pathog 15(8): e1007995.
- Duprex et al. (2002). Modulating the Function of the Measles Virus RNA-Dependent RNA Polymerase by Insertion of Green Fluorescent Protein into the Open Reading Frame. J Virol. 76(14): 7322–7328.
- Guseva et al. (2019). The Nucleoprotein and Phosphoprotein of Measles Virus. Front Microbiol. 10:1832.
- Gutsche et al. (2015). Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science. 348(6235):704–7.