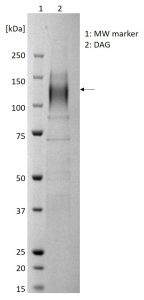
SDS-PAGE showing purified DAG protein (indicated by arrow).
Human DAG, Fc-Tag
Price range: $786.17 through $2,760.00 excl. VAT
Recombinant human alpha-dystroglycan (DAG) protein produced in HEK293 cells. Protein contains a C-terminal Fc-tag.
ALPHA-DYSTROGLYCAN (DAG) – RECOMBINANT PROTEIN
Recombinant human alpha-dystroglycan (DAG) protein produced in HEK293 cells. DAG is a major cellular receptor for Lassa Fever virus (LAFV).
PRODUCT DETAILS – ALPHA-DYSTROGLYCAN (DAG) – RECOMBINANT PROTEIN
- Recombinant human alpha-dystroglycan (DAG) – NCBI accession number NP_004384.4, amino acids 28-651.
- C-terminal transmembrane domain and the cytoplasmic tail replaced by 15 aa Glycine-Serine linker and human IgG1 Fc-tag.
- Produced in HEK293 cells and purified from culture supernatant.
- Stored in PBS, pH7.4.
BACKGROUND
The dystroglycan complex is involved in a number of processes including laminin and basement membrane assembly, sacrolemmal stability, cell survival, peripheral nerve myelination, nodal structure, cell migration, and epithelial polarization. Alpha-dystroglycan is an extracellular peripheral glycoprotein that acts as a receptor for extracellular matrix proteins containing laminin-G domains.
Lassa fever virus (LAFV) exhibits broad tissue tropism and its major cellular receptor is extracellular matrix receptor dystroglycan (DG). DG is a ubiquitously expressed and highly conserved receptor for extracellular matrix (ECM) proteins, where it provides a molecular link between the ECM and the actin-based cytoskeleton (Cao et al., 1998). DG is cleaved into the extracellular alpha-DG (α-DG) and membrane-anchored β-DG (Barresi and Campbell, 2006). In mammals, α-DG is also subject to complex O-glycosylation, which is essential for its function as a receptor for ECM proteins and arenaviruses (Yoshida-Moriguchi and Campbell, 2015; Kunz et al., 2005; Rojek et al., 2007). Binding of LASV depends on DG’s tissue-specific posttranslational modification with the unusual O-linked polysaccharide matriglycan. However, functional glycosylation of DG does not always correlate with viral tropism observed in vivo, suggesting the existence of alternative receptors. The broadly expressed phosphatidylserine (PS) receptors Axl and Tyro3 have also been identified as alternative LASV receptor candidates (Fedeli et al., 2018).
Alpha-dystroglycan also acts as a receptor for lymphocytic choriomeningitis virus glycoprotein and class C new-world arenaviruses (Imperiali et al., 2005, Kunz, 2009; Rojek et al., 2007). It is also the Schwann cell receptor for Mycobacterium leprae, the causative organism of leprosy, but only in the presence of the G-domain of LAMA2 (Rambukkana et al., 2008).
REFERENCES
- Barresi and Campbell (2006). Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 119(Pt 2):199-207.
- Cao et al. (1998). Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 282(5396):2079-81.
- Fedeli et al. (2018). Axl Can Serve as Entry Factor for Lassa Virus Depending on the Functional Glycosylation of Dystroglycan. J Virol. 92(5).
- Imperiali et al. (2005). O Mannosylation of alpha-dystroglycan is essential for lymphocytic choriomeningitis virus receptor function. J Virol. 79(22):14297-308.
- Kunz (2009). Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 387(2):245-9.
- Kunz et al. (2005). Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 79(22):14282-96.
- Rojek et al. (2007). Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan’s host-derived ligands. J Virol. 81(11):5685-95.
- Rambukkana et al. (1998). Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 282(5396):2076-9.
- Yoshida-Moriguchi and Campbell (2015). Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology. 25(7):702-13.