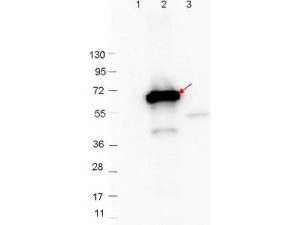
RABBIT ANTI-BORRELIA BURGDORFERI SENSU STRICTO (B31) CRASP-2 ANTIBODY
This is a polyclonal antibody, prepared against CRASP-2 from the spirochete B. burgdorferi, for use in ELISA and western blotting applications. Strain B31 is the type strain (ATCC 35210) for this organism and was derived by limited dilutional cloning from the original Lyme-disease tick isolate obtained by A. Barbour (Johnson, et al., 1984). CRASP-2 (CSPz) is a 20/21-kDa protein which binds to FHL-1 and factor H binding protein (although it interacts preferentially with Factor H) and may be predominantly expressed by serum-resistant Borrelia strains (Rossmann, et al., 2006).
PRODUCT DETAILS – RABBIT ANTI-BORRELIA BURGDORFERI SENSU STRICTO (B31) CRASP-2 ANTIBODY
- Rabbit anti-B. burgdorferi sensu stricto CRASP-2 polyclonal IgG antibody (strain B31).
- Greater than 95% purity by SDS-PAGE and buffered in 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2.
BACKGROUND
B. burgdorferi differ in their susceptibility to normal human serum and are therefore classified as complement-resistant, complement-sensitive and intermediate complement-sensitive. Complement resistant bacteria absorb human immune regulators FHL-1/reconectin and factor H using two outer surface Complement Regulator-Acquiring Surface Proteins (CRASP-1 and CRASP-2). Surface-attached FHL-1/reconectin retains its complement regulatory and cleavage activity (Kraiczy, et al., 2001). Factor H and FHL-1 serve as cofactors for factor I, a serine protease that cleaves complement component 3b (C3b) directly on the cell surface and thereby ensures resistance of spirochetes to complement-mediated lysis (Kraiczya, et al., 2007). It is possible that because of discontinuous binding regions in the factor H/FHL-1, a long-distance interaction may be involved in binding of both immune regulators. Putative coiled-coil structural elements may also be important in the interaction of B. burgdorferi CRASP-1 with factor H (Kraiczya, et al., 2007).
These proteins represent another immune evasion mechanism of B. burgdorferi, as bacteria acquire human complement regulators to control complement activation on their surface and prevent formation of toxic activation products (Kraiczy, et al., 2001).
REFERENCES
- Cordes, F. S. et al., 2005. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol. , Volume 3, pp. 276-7.
- Hallström, T. et al., 2010. • Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis., 202(3), pp. 490-8.
- Johnson, R.C., et al. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol, 34, pp. 496–497.
- Kraiczy, P. et al., 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. , 279(4), pp. 2421-9.
Immunity, 74(12), pp. 7024-7028.