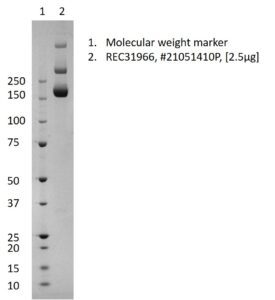
SDS-PAGE: Coomassie-stained SDS-PAGE showing purified SARS-CoV-2 stabilized spike (full-length).
SARS-CoV-2 Stabilized Spike Glycoprotein (Full-Length), His-Strep-Tag (HEK293)
Price range: $1,048.93 through $3,989.68 excl. VAT
SARS-CoV-2 Stabilized Spike (full-length) is a recombinant antigen which contains 6 mutations in S2 which stabilises the protein as a pre-fusion trimer. The protein was produced according to Hsieh et al. in HEK293 cells and purified from culture supernatant by affinity chromatography. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
SARS-COV-2 STABILIZED SPIKE GLYCOPROTEIN (FULL-LENGTH), HIS-STREP-TAG (HEK293)
SARS-CoV-2 Stabilized Spike (full-length) is a recombinant antigen which contains 6 mutations in S2 which stabilises the protein as a pre-fusion trimer. The protein was produced according to Hsieh et al. in HEK293 cells and purified from culture supernatant by affinity chromatography. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
PRODUCT DETAILS – SARS-COV-2 STABILIZED SPIKE GLYCOPROTEIN (FULL-LENGTH), HIS-STREP-TAG (HEK293)
- SARS-CoV-2 Stabilized Spike (full-length); Wuhan-Hu-1, amino acids: 1-1239, Accession: 6XKL_A
- Contains 6 mutations in S2 which stabilizes the protein as a pre-fusion trimer.
- Expressed in HEK293 and purified by affinity chromatography.
- Presented in DPBS at ~95% purity.
BACKGROUND
SARS-CoV-2 is a novel betacoronavirus and the causative agent of the COVID-19 pandemic. Coronavirus virions are covered with a spike (S) glycoprotein that binds to host-cell receptors and mediates cell entry via fusion of the host and viral membranes. Binding of the SARS-CoV-2 spike to the angiotensin-converting enzyme 2 (ACE2) receptor triggers a large conformational rearrangement of the spike from a metastable prefusion conformation to a highly stable post fusion conformation, facilitating membrane fusion. Attachment and entry are essential for the viral life cycle, making the S protein a primary target of neutralizing antibodies and an important vaccine antigen.
Recombinant expression yields of viral fusion glycoproteins has been found to be improved by prefusion stabilization, possibly by preventing triggering or misfolding that results from a tendency to adopt the more stable post fusion structure. They are also better immunogens compared to their wild-type counterparts (Pallesen et al. 2017). These variants (S-2P) contain two consecutive proline substitutions in the S2 subunit and these S2-P spikes have produced high-resolution structures by cryo-EM for SARS-CoV-2. However, even with these substitutions, the SARS-CoV-2 S-2P ectodomain is still unstable and difficult to produce reliably in mammalian cells (Walls et al., 2020; Wrapp et al., 2020).
Recently, a more stable construct (designated HexaPro), has been designed, combining multiple proline substitutions (F817P, A892P, A899P, A942P and the S-2P mutations, K986 and V987) and resulting in substantial increases in expression and stability. Large-scale HexaPro preparations retain a monodisperse SEC peak corresponding to the molecular weight of a glycosylated trimer and are indistinguishable from S-2P by negative stain EM. HexaPro expressed 9.8-fold higher than S-2P, had ~5°C increase in Tm, and retained the trimeric prefusion conformation. In addition, the binding kinetics of HexaPro to the human ACE2 receptor were shown to be comparable to those of S-2P. It remained folded in the prefusion conformation after 3 cycles of freeze-thaw, 2 days incubation at room temperature or 30 min at 55°C. HexaPro also reacts to human convalescent sera and RBD-specific mAb (CR3022) (Yuan et al., 2020) similarly to S-2P, suggesting its antigenicity is well-preserved. Collectively, these data support HexaPro as a promising candidate for SARS-CoV-2 vaccine and diagnostic development (Hsieh et al., 2020).
REFERENCES
- Hsieh et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020 Sep 18;369(6510):1501-1505.
- Pallesen et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U.S.A. 114, E7348–E7357 (2017).
- Walls et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181, 281–292.e6 (2020).
- Wrapp et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020).
- Yuan et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 368, 630–633 (2020).
You may also like…
SARS-CoV-2 (B.1.1.28/P.1) Stabilized Spike Glycoprotein (Full-Length), His-Strep-Tag (HEK293)
Price range: $1,048.93 through $3,989.68 excl. VAT
SARS-CoV-2 (B.1.1.7) Stabilized Spike Glycoprotein (Full-Length), His-Strep-Tag (HEK293)
Price range: $1,048.93 through $3,989.68 excl. VAT
SARS-CoV-2 (B.1.351) Stabilized Spike Glycoprotein (Trimeric), His-Strep-Tag (HEK293)
Price range: $1,048.93 through $3,989.68 excl. VAT