The Democratic Republic of Congo is in the grips of the second-worst Ebola outbreak it has experienced, which has now been ongoing for a year with little sign of stopping. In this blog, we discuss the important role that diagnostics play in preventing Ebola outbreaks, and how point-of-care (POC) tests are needed for more robust surveillance and outbreak management.
Diagnosing Ebola
Ebola virus disease (EVD) causes haemorrhagic fever and a host of other symptoms that are easily confused with other febrile diseases which are endemic to Sub-Saharan Africa, such as cholera and malaria. Diagnostics are therefore crucial in distinguishing Ebola infection during routine surveillance and the screening of patients. However, in past outbreaks, transmission chains have spread unchecked for weeks, before spiralling out of control – in part due to insufficient diagnostic infrastructure.
PCR or Immunoassay?
There are two types of diagnostic test used for the Ebola virus (EBOV) detection: Molecular tests are based on the polymerase chain reaction (PCR) and identify viral genomic material, whereas immunoassays detect either EBOV proteins, or antibodies raised against the virus in the blood.
PCR reference tests show very high sensitivity and specificity, making them the gold standard for accurate EBOV diagnosis. However, there are a host of practical drawbacks that have made molecular methods ineffective in widespread outbreak scenarios. During the 2014/15 outbreak, diagnosis was primarily based on RT-PCR testing of blood samples from symptomatic individuals at biocontainment laboratories by professionals with a high level of technical skill [1]. This is compounded by the need for specimen collection, logistics, and the management and reporting of data, requiring a considerable amount of time and resources to confirm cases. By the time of diagnosis, the majority of patients with confirmed EVD have been symptomatic (and infectious) for 5–6 days [2]. In fact, less than 60% of cases during the 2014/15 outbreak were diagnosed due to limited diagnostic availability [3], which in part may explain why most of the large EBOV outbreaks that have occurred have taken so long to be formally recognised. To make matters worse, without the possibility of rapid, local diagnosis, patients have often been reluctant to go to diagnostic centres due to fear of contracting disease.
Combined, these factors have led to greater interest in rapid diagnostics that can effectively detect and interrupt disease transmission [1]. More recently, rapid PCR tests have been developed using iso-thermal amplification and microfluidic techniques, but have not yet come to fruition as reliable diagnostics, and still require a moderate level of skill to use (see table below) [1].
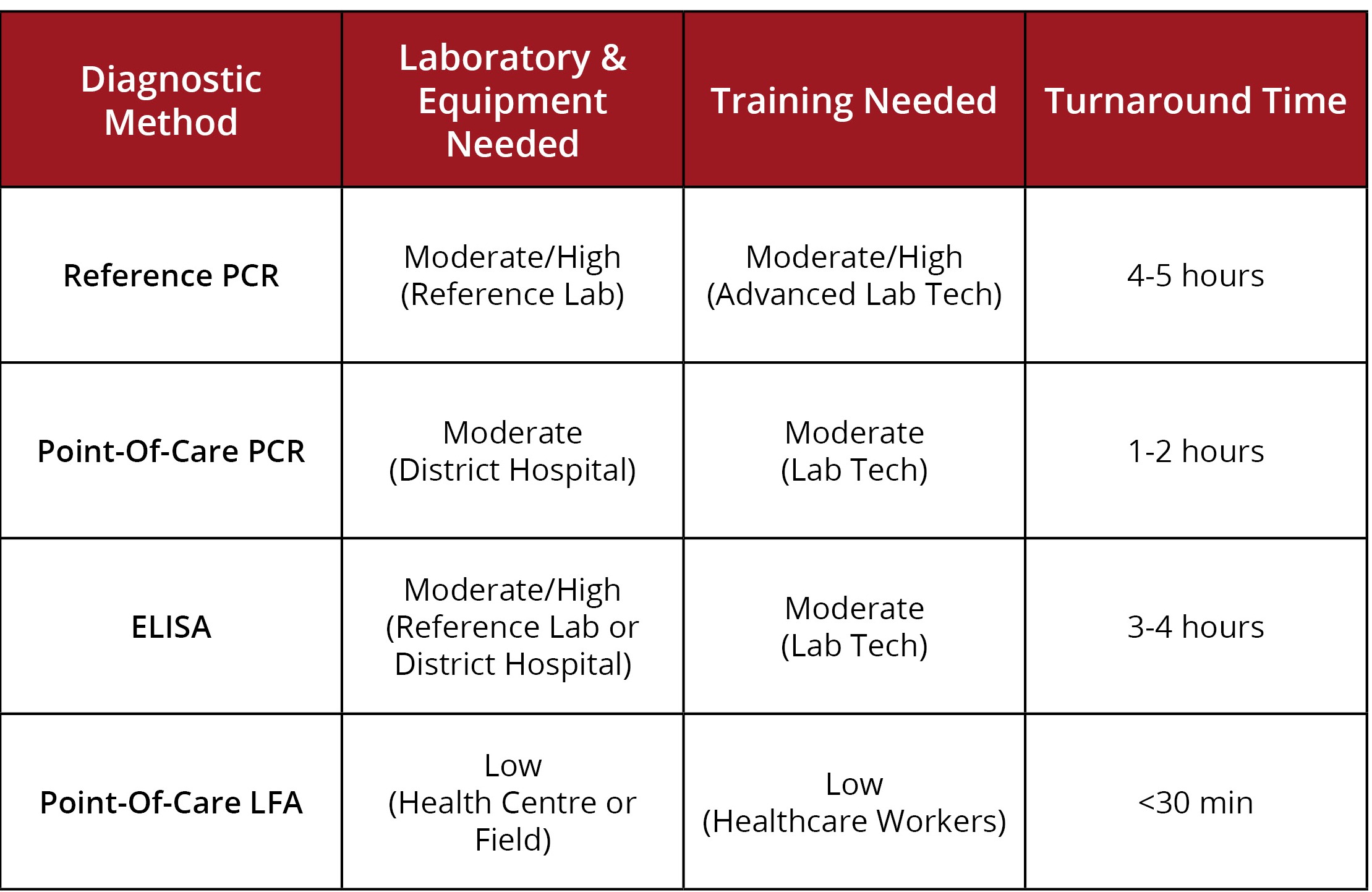
Adapted from Broadhurst et al., 2016.
Serological assays such as IgG and IgM ELISAs offer a faster, higher-throughput system for EBOV diagnosis, but equally require sterile conditions and a degree of technical skill that make them difficult to deploy for field use. Despite the challenges, field diagnostic labs have been set-up during past outbreaks with some success, but have inevitably encountered issues with logistics and turnaround times [1]. Antibody responses to EBOV infection have also been shown to vary considerably between patients, often with only short or late windows for appropriate antibody detection. A more viable alternative is the detection of viral antigens circulating in the blood, which are typically detectable within days of disease onset.
Rapid POC diagnostics
Lateral flow assays (LFA) are point-of-care diagnostics designed for single use outside laboratory settings, with at-home pregnancy tests being the best-known example. LFAs have some limitations over more-complex immunoassay procedures, such as reduced sensitivity and the potential for false-positive results when using complex sample matrices [2], but benefit from rapid time to result and ease of use. LFAs use can also be expanded for screening of those dead, or of patients with non-specific symptoms in areas of transmissions. In places without surveillance points, such as international airports, LFAs could also be used to screen patients returning from transmission zones with non-specific symptoms.
Given these advantages, LFAs are able to support more decentralised diagnostic testing capacity, allowing much swifter response to outbreaks. Rapid and accurate POC tests for Ebola could therefore drastically improve detection of infected patients.
Reagents for Rapid Diagnostics
To support the development of novel diagnostics, The Native Antigen Company provides a range of highly purified viral antigens, developed using our proprietary mammalian expression system. Our range includes envelope glycoproteins from both the Zaire and Bundibugyo strains, alongside Ebola VLPs, which contain nucleoprotein, glycoprotein and VP40 antigens. Nucleoprotein and VP40 in particular, can also be used to serologically differentiate infection from vaccination with the rVSV-EBOV vaccine, which is currently being trialled.
To complement our range of Ebola virus antigens, we also offer a range of monoclonal and polyclonal antibodies, specific to Ebola virus proteins, including Ebola glycoprotein, nucleoprotein and VP40 antigens.
Mouse Anti- Ebola Virus Envelope Glycoprotein GP (Zaire) (M922)
Price range: $454.04 through $1,139.31 excl. VAT
Sheep Anti- Ebola Virus
Price range: $369.96 through $987.96 excl. VAT
Mouse Anti- Ebola Virus Nucleoprotein Antibody (Sudan/Zaire) (7G2)
$454.04 excl. VAT
Mouse Anti- Ebola Virus VP40 Protein Antibody (Sudan/Zaire) (5B5)
$454.04 excl. VAT
Mouse Anti- Ebola Virus VP40 Protein Antibody (Sudan/Zaire) (8A1)
$454.04 excl. VAT
References
1: Broadhurst, MJ, Brooks TJ and Pollock, NR. (2016). Diagnosis of Ebola Virus Disease: Past, Present and Future. Clinical Microbiology Review.
2: Chua, AC., Cunningham, J., Moussy, F., Perkins, MD., Formenty, P. The Case for Improved Diagnostic Tools to Control Ebola Virus Disease in West Africa and How to Get There. PLoS Neglected Tropical Diseases.
3: Chen, H., Liu, K., Li, Zhao., Wang, P. Point of Care Testing for Infectious Diseases. Clinica Chimica Acta.