Rheumatoid Factor, Recombinant
The monoclonal recombinant RF is expressed in HEK293 cells as a J-chain–stabilised pentameric human IgM and diluted in human plasma. It shows native-like reactivity and is compatible across major clinical platforms, demonstrating strong recovery in multiple diagnostic systems.
PRODUCT DETAILS – Rheumatoid Factor, Recombinant
- Isotype: human IgM
- Expressed and purified from HEK293 cells
- Presented as liquid in human plasma
- The sample batch is at 6.4mg/ml.
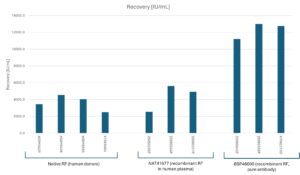
The available batches and their activities are listed below. Please note that if you require a higher activity or concentration, you may wish to consider our Rheumatoid Factor IgM, Recombinant (BSP46000), which is formulated without the addition of plasma. Alternatively, you can contact us to discuss a custom concentration tailored to your platform requirements. Please note that the final pricing will be dependent on the antibody concentration in plasma.
| Product | Batch | Concentration | Activity |
|---|---|---|---|
| NAT41677 | 25050249P | 6.4 mg/mL | 2.7 kU/mL |
| NAT41677 | 25090244P | 6.4 mg/mL | 5.6 kU/mL |
| NAT41677 | 25090257P | 6.4 mg/mL | 4.9 kU/mL |
BACKGROUND
Rheumatoid arthritis (RA) is a chronic autoimmune disorder marked by joint inflammation and systemic complications, most commonly affecting women and increasing with age [1,6]. Rheumatoid Factor (RF), a group of autoantibodies targeting the Fc region of IgG, is a key biomarker in RA diagnosis and prognosis [2,3,4]. While traditionally sourced from human sera, RF production faces challenges with consistency, supply, and biosafety. Recombinant RF provides a scalable, standardized, and pathogen-free alternative for assay development and quality control in autoimmune diagnostics. Despite its lack of disease specificity, RF remains a core component in diagnostic panels recommended by WHO and ACR [1,5,6].
REFERENCES
- World Health Organization. Rheumatoid arthritis fact sheet. WHO Website.
- Maibom-Thomsen SL, Trier NH, Holm BE, et al. Immunoglobulin G structure and rheumatoid factor epitopes. PLoS ONE. 2019;14(6):e0217624.
- Annals of the Rheumatic Diseases. 2019;78:1667–1668.
- Aho K, Heliövaara M, Maatela J, et al. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991;18(9):1282–1284.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid Arthritis Classification Criteria. Ann Rheum Dis. 2010;69(9):1580–1588.
- National Health Service (NHS) UK. Rheumatoid arthritis diagnosis. https://www.nhs.uk/conditions/rheumatoid-arthritis/diagnosis