In recent history, humanity has witnessed numerous emerging viral diseases, including the SARS, MERS, and SARS-2 coronaviruses, as well as HIV, Zika, Ebola, and H1N1 and H3N2 influenza. None have tested the resilience of our health and economic systems like the COVID-19 pandemic, which as of January 20, 2022, has infected more than 330 million people worldwide, resulting in more than 5 million deaths.
With urbanization, deforestation, intensive agriculture, livestock rearing, climate change, and globalization presenting countless opportunities for spillover and transmission, our interconnected world has made us more vulnerable to the emergence and spread of novel pathogens.
To address these challenges, investments in public health, rapid manufacturing, and the fields of virology and metagenomics are seeking to better prepare us for the next disease X. However, addressing a yet unknown threat makes for a difficult challenge. Moreover, the long time frames required to develop countermeasures, combined with the propensity of viruses to mutate and develop resistance, have made containing epidemics a challenge.
With these challenges in mind, researchers are seeking to develop a new generation of prophylactic and therapeutic technologies that can provide broader and preemptive protection.
In particular, vaccines have shown themselves to be highly cost-effective in this regard. One concept that has gained considerable attention is the universal vaccine (UV): formulations that protect against any variant or strain within a given viral family.
The how and why of universal vaccines
Most modern vaccine design strategies seek to raise neutralizing antibodies against a virus’s surface proteins, which mediate its entry to host cells. Yet, as recent SARS-CoV-2 variants have shown, the ability for these proteins to mutate can quickly change the efficacy of such vaccines. Another good example is influenza viruses, where the problem is even more pronounced as antigenic drift and shift lead to regular haemagglutinin and neuraminidase mutations that require yearly vaccine reformulation.
To address this issue, UVs take a radically different approach by instead seeking to induce protection against a broad range of target antigens—most commonly via two main strategies:
- vaccine formulations that include highly conserved antigens that are less susceptible to mutation (e.g., stalk domains), or
- vaccine formulations that include multiple variants of an antigen (e.g., chimeric antigens, as shown in Figure 1).
By stimulating broader immune responses against more conserved and/or diverse epitopes, heterotypic protection can be induced to protect against existing and future variants of a virus.
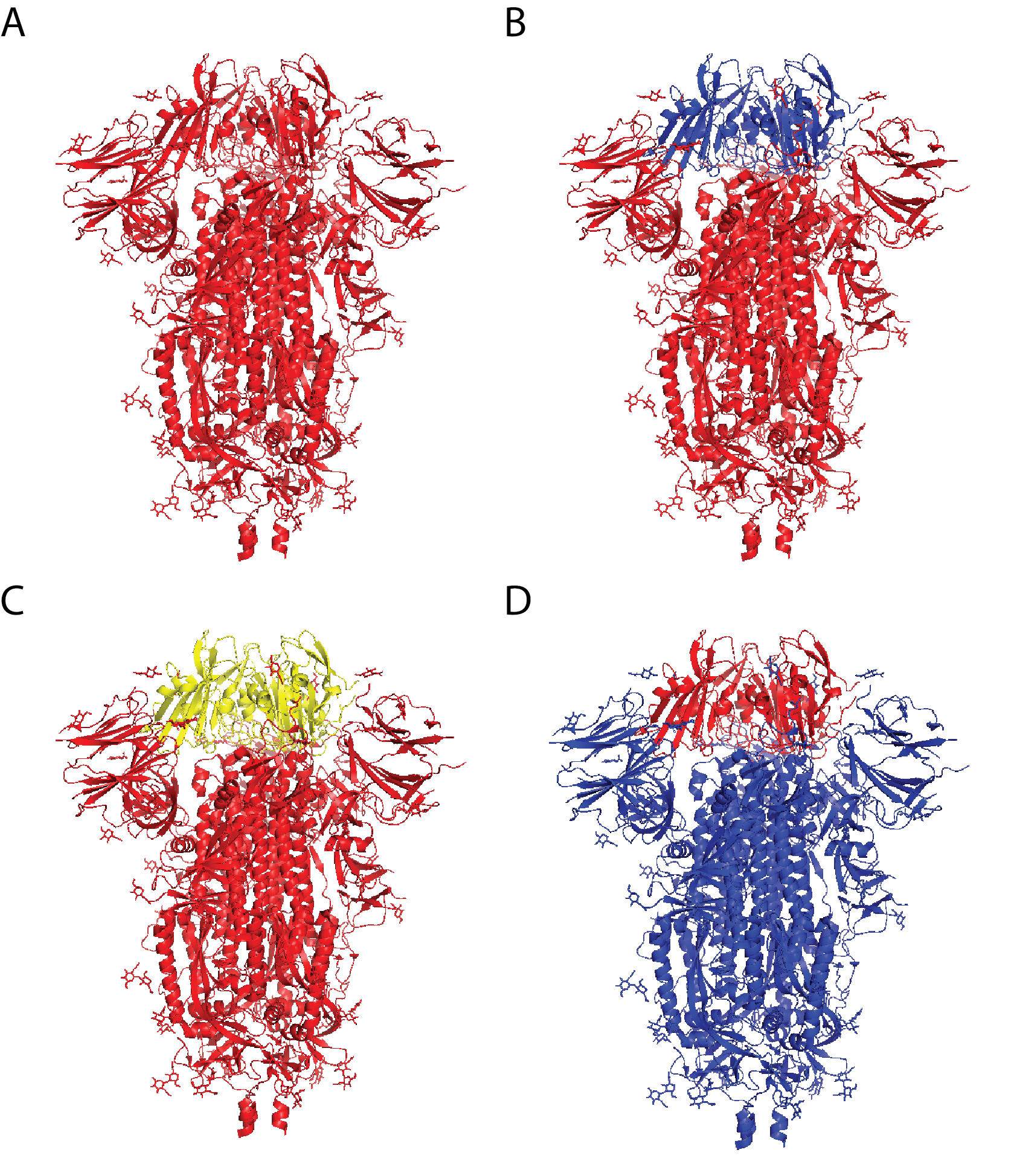
Figure 1: Chimeric coronavirus spike proteins comprising sarbecovirus vaccine by Martinez and colleagues. A) SARS-CoV-2 spike protein; B) SARS-CoV-2 spike protein with SARS-CoV receptor-binding domain (RBD); C) SARS-CoV-2 spike protein with RsSHC014 (bat coronavirus) RBD; D) SARS-CoV spike protein with SARS-CoV-2 RBD.
Immunological challenges
While UVs show promise, their development has been less than straightforward. Due to a complex range of immune phenomena, such as original antigenic sin (where the immune response is limited by the first infection and unable to mount more effective responses in subsequent infections), the breadth of cross-protection conferred by UV candidates has so far been limited to small groups of viruses with less-than-ideal heterotypic protection.
Take influenza, for example, a model virus for UVs that has so far received the majority of funding. Despite having undergone testing in human trials since the early 2000s, the first UV candidate for influenza reached a Phase 3 trial in 2018, only to show insufficient efficacy. Exacerbating the issue, the combination of long development cycles and high attrition rates of vaccine candidates has made investment in UVs relatively scarce.
However, it’s not all bad news. Considerable progress has since been made toward first-generation UVs, spurred on by a string of early-phase SARS-CoV-2 candidates and the Coalition for Epidemic Preparedness Innovations’s recent $200 million dollar commitment to funding. Moreover, SARS-CoV-2 and many other viruses may make for more viable targets than influenza due to their comparatively limited ability to mutate.

