This article was written by our friends at Infectious Diseases Hub, a free-to-access website that aims to deliver up-to-date, essential research and information on all aspects of microbiology, virology, mycology and parasitology – from bench to bedside. Infectious Diseases Hub deliver news, interviews and commentary from experts in the field, in addition to exciting multimedia content in the areas that interest you – you can sign up for free here: https://www.id-hub.com/register!
Resistant Gonorrhea
Gonorrhea is an infection caused by the sexually transmitted Neisseria gonorrhoeae, a bacterium that has progressively developed resistance to the antibiotic drugs prescribed to treat it. This started with sulphonamide resistance, first identified in 1944, and over the past 70 years the pathogen has subsequently shown an ability to develop resistance to many of the antimicrobials introduced for treatment, including the penicillins, erythromycin and tetracycline [1].
Fluoroquinolone-resistant gonorrhea has since become wide-spread and in 2007 these antibiotics were no longer recommended for treatment leaving cephalosporin antibiotics as the primary treatment option [1,2]. However, resistance to cefixime – and more rarely to ceftriaxone – has now been reported in over 50 countries, leading to updated global treatment recommendations advising a combination of ceftriaxone and azithromycin [2].
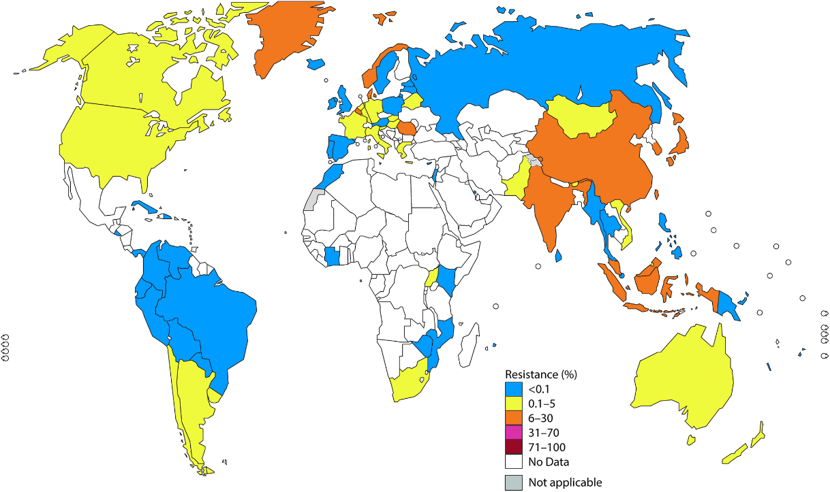
Growing concern about antimicrobial resistance culminated in 2018 when a strain resistant against first-line treatment – dual azithromycin and ceftriaxone therapy – as well as most other gonorrhea antimicrobials was identified in the UK and Australia [4]. Cases have since been reported in France, Canada and Denmark [4].
Considering the very real prospect of growing cases of untreatable gonorrhea, the need for new drugs to combat this sexually transmitted disease is pressing. Here, we take a look at some of the treatment options currently moving through the clinical pipeline, highlighting recent trial results and the next steps towards approval and clinical use.
Zoliflodacin
One of the most promising new agents being developed to treat gonorrhea is zoliflodacin, a first-in-class antibiotic under development by Entasis Therapeutics, with support from the Global Antibiotic Research and Development Partnership (GARDP). Zoliflodacin acts via inhibition of bacterial type II topoisomerases and has demonstrated positive results in a Phase II trial. In this study, the successful treatment of both urogenital and rectal gonococcal infections was reported, including microbiological cure in 98% of patients receiving a 2,000mg dose of zoliflodacin and 96% in those receiving 3,000mg. This was comparable to the control group, who received a 500mg intramuscular dose of ceftriaxone and demonstrated a 100% cure rate. However, it was noted that the drug was less effective in the treatment of pharyngeal gonococcal infections [5].
Entasis and GARDP have now announced the initiation of a global, Phase III trial to assess zoliflodacin in the treatment of uncomplicated gonorrhea. The trial, which is currently recruiting, will aim to enroll over 1,000 adults with urogenital gonorrhea from sites in the USA, Thailand, South Africa and the Netherlands [6]. Participants will be randomized to receive either zoliflodacin or a combination of ceftriaxone and azithromycin, and will be assessed after 1 week for persistence of the infection [6,7]. Data from the trial is expected in 2021.
Gepotidacin
Gepotidacin is another first-in-class antibiotic that has completed Phase II trials and is moving into Phase III studies. The compound is part of a class of antibiotics termed the triazaacenaphthylene bacterial topoisomerase inhibitors and is being developed by GlaxoSmithKline.
The progress into Phase III development follows positive results from two Phase II studies evaluating gepotidacin for the treatment of urogenital gonorrhea and acute bacterial skin and skin structure infections (ABSSSI). The first study assessed adult participants with uncomplicated urogenital gonorrhea, demonstrating that a single dose of gepotidacin achieved microbiological cure in 96% of the urogenital infections (66/69 patients) [8]. The ABSSSI study reported that two of the three doses of gepotidacin tested met the success criteria in terms of both efficacy and safety profile [9].
The Phase III program began in late 2019 and comprises two studies; the first will compare gepotidacin to ceftriaxone plus azithromycin in approximately 600 patients with urogenital gonorrhea, the second will compare gepotidacin to nitrofurantoin in 1,200 patients with uncomplicated urinary tract infection [10,11]. This second study hopes to demonstrate the potential of the compound to have activity against not just N. gonorrhoeae but a range of pathogens. Both studies are currently recruiting, with estimated completion dates in 2021 [11,12].
Testing Existing Antibiotics
Finally, with no alternative antibiotic treatments for gonorrhea and few options for new drugs in the pipeline, another option is to assess existing antibiotics for activity against this infection.
A trial published in 2019 assessed the effectiveness of gentamicin combined with azithromycin as an alternative to ceftriaxone and azithromycin for the treatment of gonorrhea [13]. The trial took place at 14 sexual health clinics across England with 720 participants randomized to either receive gentamycin injections or intravenous ceftriaxone, alongside oral azithromycin. The results demonstrated that 98% of patients receiving ceftriaxone–azithromycin were cured of their infection, compared with 91% in the gentamycin–azithromycin group. However, in genital gonorrhea gentamycin–azithromycin had a cure rate of 94%, suggesting it could present an alternative treatment for these infections [14].
In addition to this, the Public Health Service of Amsterdam and collaborators from Universiteit van Amsterdam are currently assessing three existing antibiotics in the treatment of uncomplicated anogenital gonorrhea: ertapenem (currently used to treat severe infections of the skin, lungs, stomach, pelvis, and urinary tract), fosfomycin (currently used to treat uncomplicated urinary tract infections) and gentamicin (currently used to treat bacteremia, urinary tract infections, chest infections, severe neonatal infections and other serious systemic infections).
The Phase III trial is recruiting up to 350 participants. Individuals were originally randomized to one of four treatment arms: 500mg ceftriaxone, 1,000mg ertapenem, 5mg/kg gentamicin or 6g fosfomycin. However, following interim analysis in October 2018, and the advice of the independent Data and Safety Monitoring Board, the fosfomycin arm was dropped and the trial is continuing with the remaining three arms. The study is expected to reach completion in December 2020 [15].
What’s Next?
In conclusion, there are some promising results for new agents to treat gonorrhea, with gepotidacin and zoliflodacin reaching Phase III trials; however, positive results from these studies can’t be guaranteed. In addition, depending on use and stewardship practices, new antibiotics may only have a finite lifetime – N. gonorrhoeae is a highly human-adaptive bug with propensity to developing resistance mechanisms.
In light of this, some researchers are also looking into alternative approaches to treatment – for example, host-directed therapies or the use of bacteriophages [16]. However, others argue that prophylaxis might be a better approach to this disease, shortcutting the bacteria–host–drug arms race by preventing the infection to begin with. Although further from clinical use when compared with some of the new antibiotics, there have been calls for a vaccine to protect against gonorrhea, and many teams are researching this angle for N. gonorrhoeae and other multidrug-resistant organisms [17,18].
Looking to the future, perhaps we will see new drugs and a vaccine on the horizon, but in mean-time, with the rising challenge of resistance, there is a significant need for new tools to ensure better prevention and treatment, earlier diagnosis and more complete tracking and reporting of new infections, antibiotic use, resistance incidence and treatment failures.
References
- https://www.id-hub.com/2019/07/18/gonorrhea-timeline-resistance-mechanisms/
- https://www.who.int/news-room/detail/07-07-2017-antibiotic-resistant-gonorrhoea-on-the-rise-new-drugs-needed
- https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002344
- https://www.ecdc.europa.eu/en/news-events/extensively-drug-resistant-gonorrhoea-risk-further-dissemination-within-and-across
- https://www.ncbi.nlm.nih.gov/pubmed/30403954?dopt=Abstract
- https://gardp.org/news-resources/gardp-and-entasis-therapeutics-initiate-global-phase-3-trial-of-zoliflodacin-a-first-in-class-oral-antibiotic-for-the-treatment-of-gonorrhoea/
- https://clinicaltrials.gov/ct2/show/NCT03959527?cond=Gonorrhea&draw=3&rank=4
- https://www.ncbi.nlm.nih.gov/pubmed/29617982?dopt=Abstract
- https://doi.org/10.1128/AAC.02095-16
- https://www.gsk.com/en-gb/media/press-releases/gsk-starts-a-phase-iii-clinical-programme-for-a-potential-first-in-class-antibiotic-gepotidacin/
- https://clinicaltrials.gov/ct2/show/NCT04010539?cond=Gonorrhea&draw=3&rank=1
- https://clinicaltrials.gov/ct2/show/NCT04020341?term=Gepotidacin&draw=2&rank=4
- https://www.id-hub.com/2019/05/02/gentamicin-azithromycin-combination-effective-drug-resistant-gonorrhea/
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32817-4/fulltext
- https://clinicaltrials.gov/ct2/show/NCT03294395?cond=Gonorrhea&draw=2&rank=5
- https://www.id-hub.com/2019/12/05/alternatives-antibiotics-drug-resistant-gonorrhea-interview-jenn-edwards/
- https://www.futuremedicine.com/doi/full/10.2217/fmb-2017-0262
- https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(17)30364-9/fulltext