The Native Antigen Company manufactures high-quality Pertussis toxin in its native format for a range of applications and is offering a 30% discount* on this product for the remainder of 2019. In this blog we explain the structure, mechanism-of-action and applications of Pertussis toxin in research and medicine.
Pertussis toxin
Bordetella pertussis is a gram-negative, aerobic bacterium that causes whooping cough. Infection by B. pertussis mostly occurs in children who have not yet been immunised or have lost immunity in their teens. The symptoms of infection are similar to a common cold, including a runny nose, sneezing, cough and fever. If symptoms worsen, a more severe cough with a whooping sound is common, hence the name. Pertussis toxin (PTX) is a key toxin produced by B. pertussis to modulate immune responses that aid invasion and immune evasion [1].
PTX is a 105kDa oligomer composed of 6 subunits (named S1-5, with two copies of S4), making it one of the most complex bacterial toxins identified [2]. The subunits are organised in an A-B structure, whereby S1 comprises the enzymatic A domain and subunits S2-5 comprise the B domain in a pentameric ring.
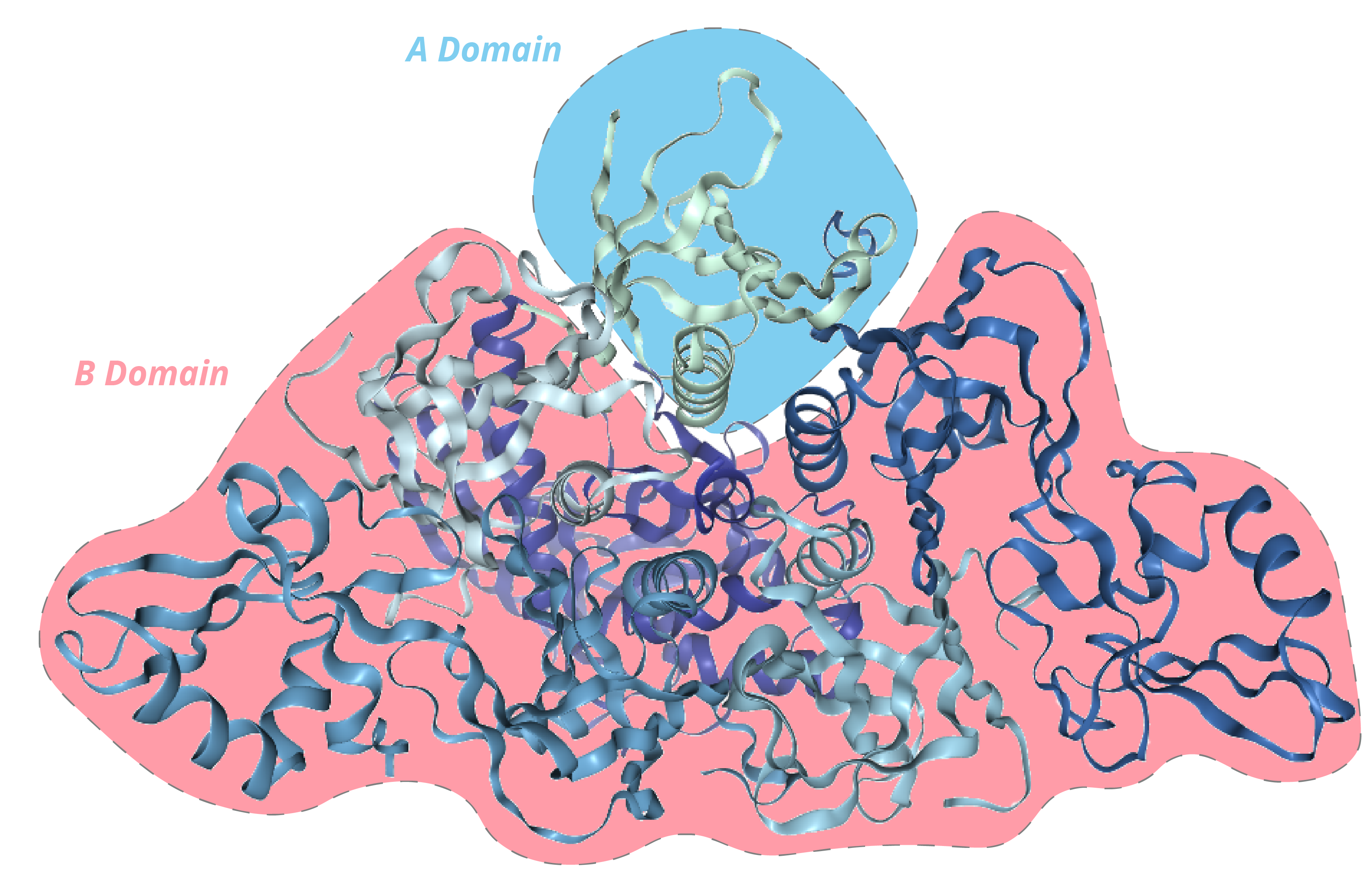
How does it work?
Subunits 1-5 are encoded by a polycistronic operon under the control of a promoter, driven by a two-component regulatory system that is responsible for regulating most of B. pertussis’s virulence factors [2]. In vitro, PTX has shown to be activated by magnesium sulphate and temperatures above 37oC which mimic the conditions of host infection. Once transcribed and assembled through an intricate pathway, PTX is actively secreted through a Ptl secretion system, where it is thought to bind non-specifically to various mammalian cell-surface receptors [3]. After endocytosis, PTX is transported via a retrograde pathway to the endoplasmic reticulum where S1 detaches and translocates to the cytosol [4]. PTX’s S1 subunit is a member of the ADP-ribosylating family of toxins, which function by transferring an ADP-ribose moiety from cellular NAD+ to intracellular host proteins, resulting in their inactivation and subsequent cellular dysfunction. S1 functions by selectively ADP-ribosylating the a-subunit of heterotrimeric G-proteins, which regulates membrane-bound adenylate cyclase [5]. As a result of this, adenylate cyclase activity is no longer inhibited, allowing the enzyme to function unrestricted and increase intracellular levels of cAMP.
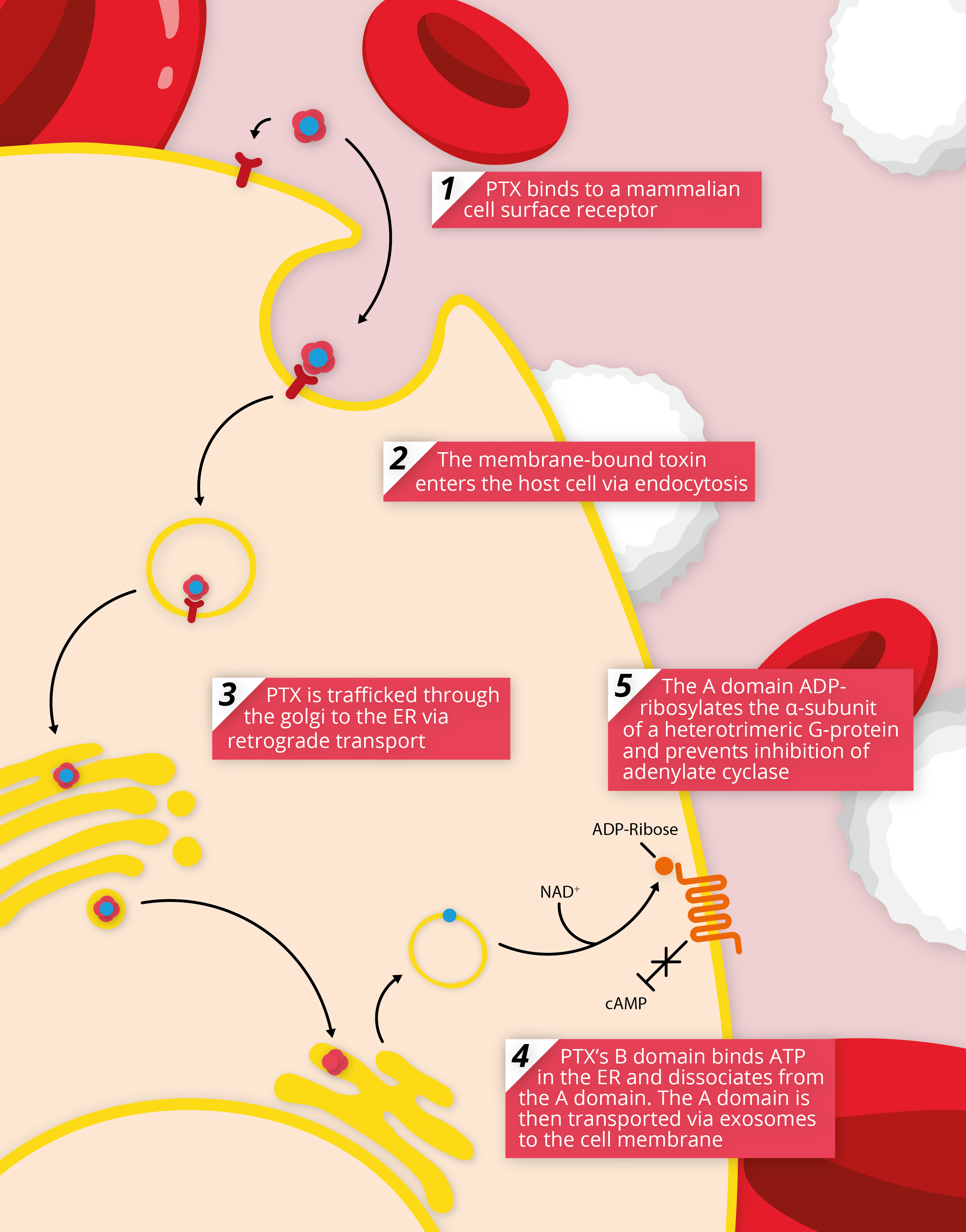
By modulating cAMP activity, PTX is able to disrupt cellular signalling mechanisms and prevent a robust immune response. One of the common effects of B. pertussis infection is a build-up of cAMP in phagocytes, which inhibits typical immune responses to bacterial infection, such as phagocytosis and induction of nitric oxide synthesis. Therefore, in addition to preventing migration to the site of infection, PTX limits the phagocytic potential of monocytes in the airways [6]. Various G-protein substrates have been identified as targets of PTX, so that almost all eukaryotic cells are affected by the toxin, which explains PTX’s to modulate a wide variety of pathways, causing wide-ranging effects that include leucocytosis, hypoglycaemia and histamine sensitivity [2].
Research applications
Cell biology
PTX is an important tool in cell biology owing to its ability to inhibit G-protein receptor pathways and is used in various research applications. Together with recently developed agents such as G-protein-directed antisera and cDNA-encoding G-proteins, PTX can be used to investigate the structure-function relationship of many receptor-G-protein cell interactions [7].
Vaccines
Early vaccine research in the 1960s identified PTX as a crucial antigen and attenuated vaccines that used PTX showed substantially improved safety profiles over wild-type pertussis vaccines and comparable efficacy. As a result, PTX is now a component of all current vaccines against whooping cough [8]. However, vaccine escape mutants with altered toxin expression have recently been isolated in countries with high vaccination coverage, requiring new and improved Pertussis vaccines to be developed [8].
Therapeutics
Due to PTX’s ability to modulate the immune response in a range of different cell types, it has been studied for use as a therapeutic to treat a number of human diseases.
Studies have shown that PTX can be used to inhibit entry and replication of HIV-1. PTX interferes with HIV pathogenesis via two distinct mechanisms: Blocking R5 HIV-1 entry through desensitisation of the CCR5 cell surface receptor, and attenuation of viral protein synthesis through inhibition of LTR-mediated transcription, independent of NF-κB activation. However, whether PTX can be used as a therapeutic agent for HIV infection is not yet clear [9].
PTX has shown to have both enhancing and inhibitory effects on experimental autoimmune diseases. For example, regular injections of PTX in mice have shown to induce the proliferation cytokines that stimulate the production of regulatory T-cells that can protect mice from the development of autoimmune diseases such as multiple sclerosis. However, PTX has also shown to induce experimental autoimmune encephalomyelitis (EAE), whereby activated T lymphocytes demyelinate axons of the CNS. Whether B. pertussis can induce the effects of autoimmune diseases in vivo has not been proven, but the evidence suggests that the bacteria could be a contributing factor to the incidence of autoimmune disease in human populations [10].
Pertussis toxin from The Native Antigen Company
The Native Antigen Company manufactures native Bordetella pertussis toxin in response to the need for highly specific, cost-effective antibody capture systems in monitoring vaccination programmes. Academic groups are also a major user of our PTX as a research reagent, which we provide through our worldwide distributors.
Our B. pertussis is grown in batch culture, then the supernatant is harvested and concentrated. Several rounds of chromatography are used to purify the native toxin to a very high level, and with minimal lot-to-lot variation. Activity of every batch of the toxin is measured in a CHO cell assay to ensure consistent performance. FHA, a major cause of false results, is removed from preparations by two rounds of chromatography. This produces a toxin that is >98% pure, and is activity tested in a CHO cluster assay.
We offer PTX in 50μg, 100μg, 250μg and 500μg vial sizes, and in lyophilized, glycerol or liquid formulations depending on customer needs. For bulk quantities up to 100mg, we offer a liquid version of the toxin which saves the need for resuspension and performs maximally in ELISAs.
We are offering a 30% discount* on our pertussis toxin for the remainder of 2019. Just use the code PTX2019 when making your next purchase. For more information on our Pertussis toxin, click on the button below:
References
1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626579/
2) https://www.ncbi.nlm.nih.gov/pubmed/21740523
3) https://www.ncbi.nlm.nih.gov/pubmed/26394801
4) https://www.ncbi.nlm.nih.gov/pubmed/18201245/
5) https://www.ncbi.nlm.nih.gov/pubmed/6296122
6) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC98857/
7) https://www.sciencedirect.com/science/article/pii/B9780121852665500312
8) https://www.ncbi.nlm.nih.gov/pubmed/25689536
9) https://www.jimmunol.org/content/166/3/1863
10) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851156/
- Discount available on standard size catalogue products only, and cannot be combined with any other discounts or promotions