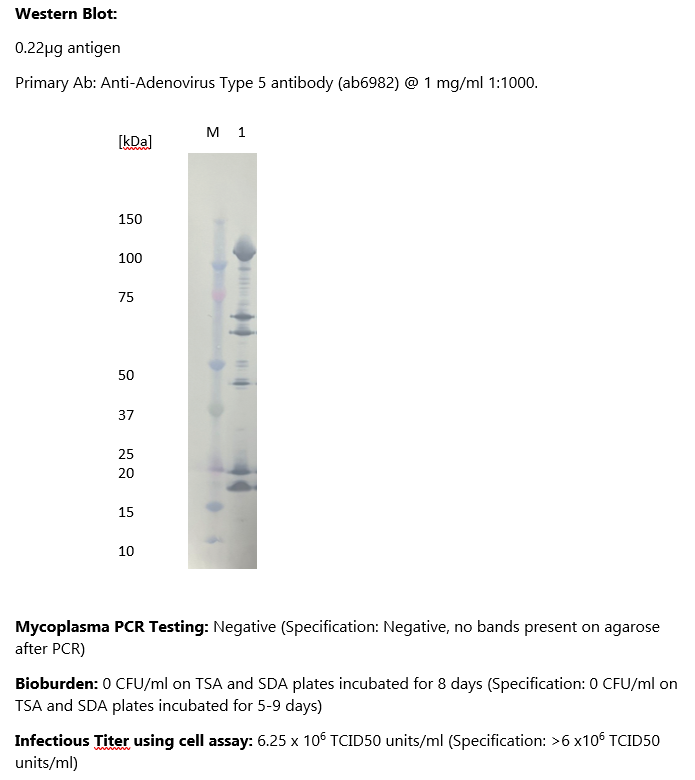
Adenovirus Type 5, Control Grade
Our Control Grade Ad5 (Mastadenovirus h5) have similar features to our Wild-Type Ad5 Particles produced in HEK293 cells, with enhanced quality analysis and characterisation making it a suitable viral reference material.
PRODUCT DETAILS – Adenovirus Type 5, Control Grade
- Control grade Adenovirus Type 5 particles.
- 50ul vial, Buffer: 20mM Tris, 25mM NaCl, 2,5% Glycerol, pH = 8,0
- Double CsCl gradient purification with DNase treatment and dialysis.
- Produced in HEK293 cells and stored in pH 7.8 PBS buffer.
- Physical titer: >1 x 10e12 particles per ml
- Infectious titer : > 1 x 10e7 TCID50 units/ml
- Mycoplasma testing by PCR
- UV A280/260 ratio between 1.2 and 1.4
- Sterility / Adventitious agents testing on permissive plates
- Minimal lot-to-lot variation
Please contact us to discuss your batch size requirements, or to tailor bespoke QA & specifications.
BACKGROUND
Adenoviruses are medium-sized (80–100 nm), non-enveloped viruses. They have an icosahedral nucleocapsid containing a linear, double-stranded DNA genome of approximately 36 kb (Nermut, 1984). The viral genome is grouped into different transcriptional units, designated early (E1, E2, E3, E4), intermediate, and late. The E1 gene is essential for activation of other viral genes and for viral replication. Deletion of the E1 gene results in viruses that are replication incompetent in normal cells. However, replication-competent viral particles can be produced from E1-deleted viral vectors by providing the E1 gene in trans. The E3 gene is nonessential for either viral replication or infection (Flint, 1999).
Wild-type adenoviruses, including human adenovirus type 5, are associated with a number of mild disorders, such as lower respiratory tract infections in the elderly or children. Of the 57 human adenovirus serotypes, 5 (Ad5) is one of the best studied, providing a better understanding of virus biology, host cellular processes and virus-cell interactions during infection. Ad5 is also widely used for transgene delivery in preclinical and clinical gene therapy studies; replication-defective vectors have been described expressing Ebola glycoproteins and malaria antigens for vaccine trials.
REFERENCES
- Flint, J., 1999. Organization of the adenoviral genome. In: P. Seth, ed. Adenoviruses: Basic Biology to Gene Therapy. Austin, TX, USA: R.G. Landes Company, pp. 17-30.
- Nermut, M. V., 1984. The Architecture of Adenoviruses. In: . (eds) . In: H. S. Ginsberg, ed. The Adenoviruses. The Viruses. Boston, MA: Springer.
- Thoma, C. et al. 2013. Adenovirus Serotype 11 Causes Less Long-Term Intraperitoneal Inflammation than Serotype 5: Implications for Ovarian Cancer Therapy. Virology, 74-83. Elsevier.
- Dyer, A. et al. 2017. Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators. Mol Ther Oncolytics, 18-30. Elsevier.
This material is intended for research purposes only, NOT FOR HUMAN USE. Handle material as a category 2 pathogen using category 2 facilities and procedures.