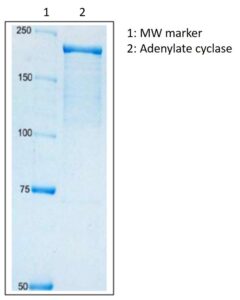
SDS-PAGE: SDS polyacrylamide gel electrophoresis (8% gel) of adenylate cyclase toxin.
Bordetella Pertussis Adenylate Cyclase, Active
$687.39 excl. VAT
Adenylate cyclase is a toxin produced by a gram-negative coccobacillus, Bordetella pertussis. This toxin penetrates into the animal cells and produces cAMP from ATP in the cytoplasm and can be used for functional studies on the cAMP- signaling pathway.
BORDETELLA PERTUSSIS ADENYLATE CYCLASE, ACTIVE
Adenylate cyclase is a toxin produced by a gram-negative coccobacillus, Bordetella pertussis. This toxin penetrates into the animal cells and produces cAMP from ATP in the cytoplasm. It belongs to the RTX family of toxins produced by many gram-negative bacteria.
PRODUCT DESCRIPTION – BORDETELLA PERTUSSIS ADENYLATE CYCLASE, ACTIVE
- Recombinant toxin of Bordetella pertussis Tohama strain.
- Expressed in E. coli from the structural gene cyaA and its activator gene cyaC.
- Purified by calmodulin-sepharose 4B chromatography (Friedman, 1987)
- Greater than than 90% purity by SDS-PAGE.
- Addition to medium significantly increased levels of cellular cAMP in CHO and human monocyte cells.
BACKGROUND
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Gene analysis has shown that adenylate cyclase is synthesized as a large precursor of 1706 amino acids. The protein consists of three domains: from the N-terminus up to roughly residue 400, there is an adenylate-cyclase domain; between residues 500 and 700, there is a hydrophobic domain; and from residue 1000 to the C-terminus, there are calcium binding repeats. The calmodulin-stimulated catalytic activity resides in the amino-terminal 400 amino acids whereas the 1300 amino acid carboxy-terminal part of the precursor is endowed with haemolytic activity. The catalytically active 43 kDa form of adenylate cyclase is organized in two domains: the N-terminal domain of 25 kDa harbors the catalytic site, and the 18 kDa C-terminal domain carries the main calmodulin-binding site (Glaser et al., 1988).
The toxin is secreted by the Type I secretion system, which spans both membranes and periplasm space, allowing it to be secreted from the cytoplasm directly outside the cell which then binds to target cells by the complement receptor 3 (CD11b/CD18, or Mac-1). These properties have been exploited to engineer the toxin as a potent non-replicating vector able to deliver antigens into antigen presenting cells and elicit specific cell-mediated immune responses. Antigens of interest can be inserted into the toxin protein to yield recombinant molecules that are targeted in vivo to dendritic cells, where the antigens are processed and presented by the major class I and class II histocompatibility complexes (MHC-I and II) (Chenal et al., 2018).
REFERENCES
- Chenal A, Ladant D. Bioengineering of Bordetella pertussis Adenylate Cyclase Toxin for Antigen-Delivery and Immunotherapy. Toxins (Basel). 2018 Jul 20;10(7):302.
- Friedman RL (1987) Bordetella pertussis adenylate cyclase: isolation and purification by calmodulinsepharose 4B chromatography. Infect Immun 55:129-134.
- Glaser P, Danchin A, Ladant D, Barzu O, Ullmann A. Bordetella pertussis adenylate cyclase: the gene and the protein. Tokai J Exp Clin Med. 1988;13 Suppl:239-52.