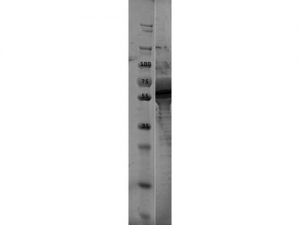
SDS-PAGE of OspE Protein. Lane 1: Molecular Weight Marker. Lane 2: OspE Protein. Load: 10 µl at 1:4 dilution. Predicted/Observed size: 59.5 kDa fusion protein, 17.1 kDa for OspE, 42.4 kDa for MBP alone.
Borrelia burgdorferi sensu stricto (B31) ErpN/OspE Protein
$881.36 excl. VAT
Recombinant Borrelia burgdorferi ErpN/OspE protein fused to MBP-tag and produced in E. coli.
BORRELIA BURGDORFERI SENSU STRICTO (B31) ERPN/OSPE PROTEIN
Recombinant Borrelia burgdorferi ErpN protein, fused to an MBP-tag and produced in E. coli (>90% purity).
PRODUCT DETAILS – BORRELIA BURGDORFERI SENSU STRICTO (B31) ERPN/OSPE PROTEIN
- Recombinant Borrelia burgdorferi sensu stricto (B31) ErpN/OspE (NCBI Accession Number: NP_051416.1).
- Greater than 90% pure (by SDS-PAGE) in 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 and 0.01% (w/v) Sodium Azide.
BACKGROUND
ErpN (OspE/F-Related Protein D), also known as Arp37, is encoded by the spirochete Borrelia burgdorferi, which is carried by Ixodes ticks. Strain B31 is the type strain (ATCC 35210) for this organism and was derived by limited dilutional cloning from the original Lyme-disease tick isolate obtained by A. Barbour (Johnson, et al., 1984). Several studies have demonstrated that infected humans and animals produce antibodies directed against Erp proteins within the first 2-4 weeks of infection, suggesting Erp synthesis occurs during the initial stages of vertebrate infection. Surface-exposed Erp proteins may then facilitate interactions with host tissues during the establishment of vertebrate infection (Fraser, et al., 1997).
Erp proteins from B. burgdorferi are believed to be lipoproteins, based on their predicted amino acid sequences. The spirochete migrates from the tick midgut during feeding to its salivary glands and are thus transmitted to the mammal host. This transition may be facilitated by changes in expression of some B. burgdorferi genes. It is believed that expression of the various proteins associated with the spirochete may be regulated by the changes in tick life cycle, changes in conditions during tick feeding (such as temperature, pH, and nutrients) and/or in coordination with the course of infection of the mammal host (Stevenson, et al., 1996).
REFERENCES
- Fraser, C. M. et al., 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, Volume 390, pp. 580-586.
- Johnson, R.C., et al. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol, 34, pp. 496–497.
- Stevenson, B., Tilly, K. & Rosa, P. A., 1996. A Family of Genes Located on Four Separate 32-Kilobase Circular Plasmids in Borrelia burgdorferi B31. J Bacteriol, 178(12), pp. 3508-3516.