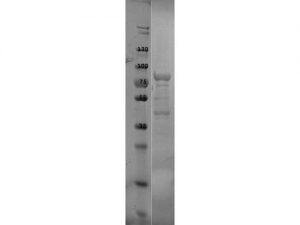
SDS-PAGE of P39 protein. Lane 1: Molecular Weight Marker. Lane 2: P39 protein. Load: 10 µl at 1:2 dilution. Predicted/Observed size: 77.8 kDa fusion protein, 35.4 kDa for p39, 42.4 kDa for MBP alone.
Borrelia burgdorferi sensu stricto (B31) P39 Protein
$881.36 excl. VAT
Recombinant Borrelia burgdorferi P39 protein fused to MBP-tag and produced in E. coli.
BORRELIA BURGDORFERI SENSU STRICTO (B31) P39 PROTEIN
This is a recombinant Borrelia burgdorferi P39 protein, fused to an MBP-tag and produced in E. coli (>90% purity).
PRODUCT DETAILS – BORRELIA BURGDORFERI SENSU STRICTO (B31) P39 PROTEIN
- Recombinant Borrelia burgdorferi sensu stricto (B31) P39 (NCBI Accession Number: NP_212517.1).
- Greater than 90% pure (by SDS-PAGE) in 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 and 0.01% (w/v) Sodium Azide.
BACKGROUND
P39 is encoded by the spirochete B. burgdorferi, which is carried by Ixodes ticks. Strain B31 is the type strain (ATCC 35210) for this organism and was derived by limited dilutional cloning from the original Lyme-disease tick isolate obtained by A. Barbour (Johnson, et al., 1984). The p39 protein, or Basic membrane protein A (BmpA), is one of the immunogenic cell membrane components displayed on the outer surface of B. burgdorferi, the spirochete carried by Ixodes ticks and is an important antigen for serodiagnosis of human infection. BmpA is involved in borrelial pathogenicity and participates in the development of borrelial arthritis. It is a member of the chromosomally-located paralogous family 36 which also includes BmpB, BmpC and BmpD. Its expression is co-regulated with that of BmpC and BmpB and may be subject to global regulation (Bryksin, et al., 2010).
The spirochete migrates from the tick mid-gut during feeding to its salivary glands and is then transmitted to the mammal host. This transition may be facilitated by changes in expression of some B. burgdorferi genes (Aron, et al., 1996). It is believed that expression of the various proteins associated with the spirochete may be regulated by the changes in tick life cycle, changes in conditions during tick feeding (such as temperature, pH, and nutrients) and/or in coordination with the course of infection of the mammal host.
BmpA and its three paralogous proteins, BmpB, C and D, all bind to mammalian laminin and BmpA-directed antibodies, significantly inhibiting the adherence of live B. burgdorferi to laminin. The laminin-binding domain of BmpA was mapped to the 80 carboxy-terminal residues. Solubilized collagen inhibited BmpA-laminin binding, suggesting interactions through the collagen-binding domains of laminin. BmpA does not bind mammalian type I or type IV collagens or fibronectin (Verma, et al., 2009).
BmpA is expressed during the invasion of the spirochete and in the evolution of the arthritis of Lyme disease in mammals. The major products of the B. burgdorferi basic membrane protein (bmp) A/B operon that are induced in murine and human joints possess inflammatory properties. Non-lipidated and lipidated versions of BmpA have been shown to induce the pro-inflammatory cytokines TNF-α and IL-1ß in human synovial cells. The induction of cytokine responses in synovial cells via activation of the NF-κB and p38 MAP kinase pathways could potentially contribute to the pathogenesis of Lyme arthritis (Yang, et al., 2008).
REFERENCES
- Aron, L., Toth, C., Godfrey, H. P. & Cabello, F. C., 1996. Identification and mapping of a chromosomal gene cluster of Borrelia burgdorferi containing genes expressed in vivo. FEMS Microbiol. Lett. , Volume 145, pp. 309-314.
- Bryksin, A. V., Tomova, A., Godfrey, H. P. & Cabello, F. C., 2010. BmpA is a surface-exposed outer membrane protein of Borrelia burgdorferi. FEMS Microbiol Lett., 309(1), pp. 77-83.
- Johnson, R.C., et al. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol, 34, pp. 496–497.
- Verma, A., Brissette, C. A., Bowman, A. & Stevenson, B., 2009. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun., 77(11), pp. 4940-6.
- Yang, X. et al., 2008. Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety. Microbes Infect., 10(12-13), pp. 1300-8.