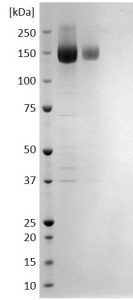
SDS-PAGE: Reducing SDS-PAGE gel showing purified CMV Glycoprotein B envelope protein (Human Fc tag) running with an expected MW of approximately 140-160kDa.
Cytomegalovirus Glycoprotein B (gB), Human Fc
Price range: $626.41 through $2,511.95 excl. VAT
Recombinant human cytomegalovirus glycoprotein B (gB) protein, comprising amino acids 1-700 from the BE/14/2011 strain and incorporating a human IgG1 Fc-tag at the C-terminus. Produced in HEK293 cells.
CYTOMEGALOVIRUS GLYCOPROTEIN B (gB), HUMAN FC
This CMV glycoprotein B recombinant protein has been manufactured in our optimised mammalian cell expression system to provide the highest quality for assay and vaccine research.
PRODUCT DETAILS – CYTOMEGALOVIRUS GLYCOPROTEIN B (gB), HUMAN FC
- Recombinant gene product from HCMV UL55 locus, amino acid 1-700 (NCBI Accession Number: AKI13965.1). Sequence strain is Human betaherpesvirus 5 Towne.
- A glycine-serine linker and human IgG1 Fc-tag was fused to amino acid 700 of gB. Amino acids 457-460 were replaced with the sequence TTQT to prevent furin cleavage of gB.
- Expressed and purified from HEK293 cells. Presented in DPBS pH7.4.
BACKGROUND
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and monkeys serve as natural hosts. There are currently eight species in this genus including the type species, Human betaherpesvirus 5 (HCMV, human cytomegalovirus, HHV-5), which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia. All herpesviruses share a characteristic ability to remain latent within the body over long periods. Although they may be found throughout the body, CMV infections are frequently associated with the salivary glands in humans and other mammals.
Cytomegalovirus glycoprotein B (gB) is an important envelope protein of Human Cytomegalovirus (HCMV), acting as a receptor for entry of the virus into fibroblasts. This protein has been the target of vaccines designed to prevent HCMV infection by preventing entry into host cells. However, the HCMV pentameric complex also acts as a receptor for host-cell entry into epithelial and endothelial cells, and vaccine results using gB alone have only shown partial success.
Currently, there is no licensed vaccine to prevent CMV infection. Therefore, attempts to reduce the risk of cCMV infection by improving awareness, behaviour, screening, diagnosis and the treatment of symptomatic cases are the most immediate means of mitigating the health burden caused by cCMV (CDC).
REFERENCES
- Centers for Disease Control and Prevention (CDC). Cytomegalovirus (CMV) and Congenital CMV Infection.