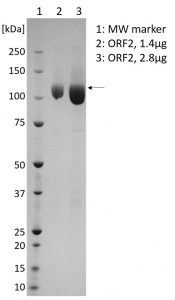
SDS-PAGE showing purified ORF2 protein (indicated by arrow).
Hepatitis E Virus Capsid Protein (ORF2), Mouse Fc-Tag
$551.29 – $1,947.37 excl. VAT
Recombinant Hepatitis E (HEV genotype 1) Capsid protein (ORF2) with C-terminal mouse Fc-tag, produced in HEK293 cells.
HEPATITIS E VIRUS CAPSID PROTEIN (ORF2), MOUSE FC-TAG
Recombinant HEV ORF2, amino acids 1-660 (Uniprot accession number Q68985.1) produced in HEK293 cells by transient transfection. A 15 amino acid glycine-serine linker followed by a mouse IgG2a Fc region (CH2-CH3) was added to the C-terminus of the protein. Hepatitis E ORF2 Fc protein was purified from culture supernatant using Protein G affinity chromatography followed by dialysis.
PRODUCT DETAILS – HEPATITIS E VIRUS CAPSID PROTEIN (ORF2), MOUSE FC-TAG
- Recombinant Hepatitis E virus Capsid Protein with C-terminal mouse IgG2a Fc-tag (NCBI Accession Number: Q68985.1).
- Protein (aa 1-660) expressed in HEK293 cells and greater than 95% purity by SDS-PAGE.
- Available in liquid format and buffered in DPBS pH7.4.
BACKGROUND
Hepatitis E virus (HEV) is a single-stranded positive-sense non-enveloped RNA virus and is a leading cause of acute hepatitis worldwide (Kamar, et al., 2017), infecting ∼20 million people annually (Smith, et al., 2014). HEV is currently the only representative of the hepevirus genus, belonging to the family Hepeviridae. Four genotypes 1-4 of the Hepatitis E virus have been identified so far, with each genotype having a different geographical distribution. HEV genotype 1 and 2 are restricted to humans and are found in Africa and Asia, whereas genotype 3 is found worldwide and genotype 4 is found in China and Japan.
The viral RNA is ∼7.2 kb in size with three partially overlapping open reading frames (Purcell & Emerson, 2001). ORF1 encodes non-structural proteins, ORF2 encodes the capsid protein (Tam & Smith, 1991) and ORF3 encodes a cytoskeleton-associated phosphoprotein (Zafrullah, et al., 1997). ORF2 is initially synthesized as a large glycoprotein precursor which is then cleaved into the mature viral capsid protein; it has also been implicated in viral replication, as it binds to the 5′ end of the viral genome (Surjit, et al., 2004).
The main HEV immune epitopes, especially neutralizing epitopes, are located on the ORF2 protein (Zhou, et al., 2016). There are two potential N-glycosylation sites in the S domain, and one in the P domain within a region that forms a protruding spike from the shell, forming a cell-attachment and neutralizing antigenic site (Xu, et al., 2016; Zhang, et al., 2016).
HEV produces three forms of the ORF2 capsid protein; infectious/intracellular ORF2 (ORF2i), glycosylated ORF2 (ORF2g) and cleaved ORF2 (ORF2c). The ORF2i protein is associated with infectious particles, whereas the ORF2g and ORF2c proteins are secreted glycoproteins, not associated with infectious particles. ORF2g and ORF2c have been shown to be the most abundant antigens detected in sera from patients (Montpellier, et al., 2018; Qi, et al., 2015). Anti-HEV antigen (Ag)-specific enzyme-linked immunosorbent assay (ELISA) directed against the HEV capsid has proven to be a useful tool in discriminating chronic from acute HEV infection (Behrendt, et al., 2016). Hepatitis E ORF2 Fc protein is therefore a promising candidate antigen for diagnostic and vaccine development.
REFERENCES
- Behrendt, P., Bremer, B. & et al., 2016. Hepatitis E Virus (HEV) ORF2 Antigen Levels Differentiate Between Acute and Chronic HEV Infection. J Infect Dis., 214(3), pp. 361-8.
- Kamar, N., Izopet, J., Pavio, N. & et al., 2017. Hepatitis E virus infection. Nat Rev Dis Primers, Volume 3, p. 17086.
- Montpellier, C., Wychowski, C. & et al., 2018. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology, 154(1), pp. 211-223.
- Purcell, R. H. & Emerson, S. U., 2001. Hepatitis E virus. In: D. M. Knipe & P. M. Howley , eds. Fields virology. Philadelphia, Pa.: Lippincott Williams & Wilkins, pp. 3051-3061.
- Qi, Y., Zhang, F. & et al., 2015. Hepatitis E Virus Produced from Cell Culture Has a Lipid Envelope. PLoS One, 10(7).
- Smith, D. B., Simmonds, P. & et al., 2014. Consensus proposals for classification of the family Hepeviridae. International Committee on Taxonomy of Viruses Hepeviridae Study Group. J Gen Virol., 95(10), pp. 2223-32.
- Surjit, M., Jameel, S. & et al., 2004. The ORF2 protein of hepatitis E virus binds the 5′ region of viral RNA. J Virol., 78(1), pp. 320-8.
- Tam, A. W. & Smith, M. M., 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology, Volume 185, pp. 120-131.
- Xu, M., Behloul, N. & et al., 2016. Role of asparagine at position 562 in dimerization and immunogenicity of the hepatitis E virus capsid protein. Infect Genet Evol., Volume 37, pp. 99-107.
- Zafrullah, M., Ozdener,, M. H. & et al., 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol., Volume 71, pp. 9045-9053.
- Zhang, X., Bilic, I. & et al., 2016. C-Terminal Amino Acids 471-507 of Avian Hepatitis E Virus Capsid Protein Are Crucial for Binding to Avian and Human Cells. PLoS One, 11(4).
- Zhou, Y., Zhao, C. & et al., 2016. Characteristics and Functions of HEV Proteins. Adv Exp Med Biol., Volume 948, pp. 17-38.