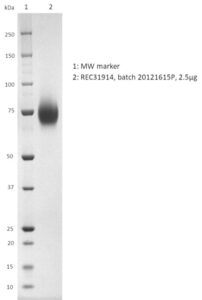
SDS-PAGE: Coomassie-stained reducing SDS-PAGE showing purified Influenza A (A/Ohio/2017) Neuraminidase (NA) protein.
Influenza A [A/Ohio/2017] Neuraminidase (NA), His-Tag
$553.29 – $1,939.44 excl. VAT
This Influenza virus neuraminidase protein is derived from the NA sequence of the IAV [A/swine/Ohio/17TOSU5163/2017], expressing aa 34-469, and fused with a polyhistidine tag at the C-terminus. The influenza virus neuraminidase protein is expressed in HEK293 cells.
INFLUENZA A [A/OHIO/2017] NEURAMINIDASE (NA), HIS-TAG
Influenza A (A/Ohio/2017) Neuraminidase is derived from the NA sequence of the IAV [A/swine/Ohio/17TOSU5163/2017], expressing aa 34-469, and fused with a polyhistidine tag at the C-terminus. The influenza virus neuraminidase protein is expressed in HEK293 cells.
PRODUCT DETAILS – INFLUENZA A [A/OHIO/2017] NEURAMINIDASE (NA), HIS-TAG
- Recombinant Influenza A (A/Ohio/2017) Neuraminidase of A/swine/Ohio/17TOSU5163/2017 (NCBI Accession Number: QBF03131.1), amino acids 34-469 and a C-terminal His-tag.
- Expressed in HEK293 cells, and purified from culture supernatant by immobilised metal affinity chromatography and dialysis.
- Presented as liquid in DPBS and greater than >95% purity by SDS-PAGE.
BACKGROUND
The influenza A viruses are negative-sense, single-stranded, segmented RNA viruses of the genus Alphainfluenzavirus, family Orthomyxoviridae. There are several subtypes, named according to the type of Haemagglutinin (H1-18) and Neuraminidase (N1-11) (Centers for Disease Control and Prevention, 2017). Humans are generally infected by influenza viruses of the subtypes H1, H2 or H3, and N1 or N2. Influenza A virus subtype H3N2 (A/H3N2) is a subtype of viruses that causes influenza (flu) and can infect birds and mammals. In birds, humans, and pigs, the virus has mutated into many strains and in years in which it is the predominant strain, there are usually more hospitalizations. The Hong Kong flu was the first known outbreak of the H3N2 strain, and the 1968- 1969 pandemic killed an estimated one million people worldwide (CDC, 2019). WHO convenes technical consultations in February and September each year to recommend viruses for inclusion in influenza vaccines for the northern and southern hemisphere influenza seasons, respectively. Flu vaccines are based on predicting which mutants of H1N1, H3N2, H1N2, and influenza B will proliferate in the next season. Separate vaccines are developed for the Northern and Southern Hemispheres in preparation for their annual epidemics. One influenza A (H1N1), one influenza A (H3N2), and one or two influenza B viruses (depending on the vaccine) are included in each season’s influenza vaccines (CDC, 2019).
REFERENCES
- Centers for Disease Control and Prevention (CDC). (2019). Influenza (Flu).
- World Health organisation (WHO). Influenza. Recommended composition of influenza virus vaccines for use in the 2020 – 2021 northern hemisphere influenza season (2020).

![Influenza A [A/Hong Kong/483/97 (H5N1)] Hemagglutinin (HA), His-Tag](https://thenativeantigencompany.com/wp-content/uploads/1970/01/shutterstock_1204546204-300x300.jpg)