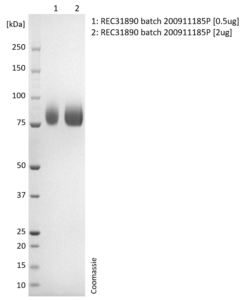
SDS-PAGE: Coomassie-stained reducing SDS-PAGE showing purified Influenza B Hemagglutinin (HA) protein.
Influenza B [B/Palermo/4/2013] Hemagglutinin (HA), His-Tag
$464.04 – $1,628.10 excl. VAT
This Influenza virus hemagglutinin protein is derived from the HA sequence of B/Palermo/4/2013, expressing aa 16-547, and fused with a polyhistidine tag at the C-terminus. The influenza virus hemagglutinin protein is manufactured in HEK293 cells to
INFLUENZA B [B/PALERMO/4/2013] HEMAGGLUTININ (HA), HIS-TAG
This Influenza virus hemagglutinin protein is derived from the HA sequence of B/Palermo/4/2013, expressing aa 16-547, and fused with a polyhistidine tag at the C-terminus. The influenza virus hemagglutinin protein is manufactured in HEK293 cells to
PRODUCT DETAILS – INFLUENZA B [B/PALERMO/4/2013] HEMAGGLUTININ (HA), HIS-TAG
- Recombinant Influenza B [B/Palermo/4/2013] Hemagglutinin (NCBI Accession Number: AMB72169.1), amino acids 16-547 and a C-terminal His-tag.
- Expressed in HEK293 cells, and purified from culture supernatant by immobilised metal affinity chromatography and buffer exchange.
- Presented as liquid in DPBS and greater than >95% purity by SDS-PAGE.
BACKGROUND
Influenza B virus is the only species in the genus Betainfluenzavirus in the virus family Orthomyxoviridae. Influenza B virus is only known to infect humans and seals and this is believed to account for the lack of associated influenza pandemics in contrast with those caused by the morphologically similar influenza A virus, although both mutate by both antigenic drift and reassortment. However, it is thought that Influenza B virus could cause significant morbidity and mortality worldwide, particularly in adolescents and schoolchildren. WHO convenes technical consultations in February and September each year to recommend viruses for inclusion in influenza vaccines for the northern and southern hemisphere influenza seasons, respectively. Flu vaccines are based on predicting which mutants of H1N1, H3N2, H1N2, and influenza B will proliferate in the next season. Separate vaccines are developed for the Northern and Southern Hemispheres in preparation for their annual epidemics. One influenza A (H1N1), one influenza A (H3N2), and one or two influenza B viruses (depending on the vaccine) are included in each season’s influenza vaccines (CDC, 2019). Since December, 2019, coronavirus disease 2019 (COVID-19) has been an international public health emergency and co-infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses have been reported, complicating their diagnosis (Azekawa et al., 2020; Cuadrado-Payán et al., 2020; Wu et al., 2020).
REFERENCES
-
- Azekawa S, Namkoong H, Mitamura K, Kawaoka Y, Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20:e00775. Published 2020 Apr 21.
- Centers for Disease Control and Prevention (CDC). (2019). Influenza (Flu).
- Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84.
- Wu X, Cai Y, Huang X, et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China. Emerg Infect Dis. 2020;26(6):1324-1326.