MAYARO VIRUS E2 ENVELOPE PROTEIN – HUMAN FC-TAG
Mayaro Virus E2 protein is a unique product that has been developed in response to the need for high purity, properly assembled and glycosylated Mayaro virus antigens for use in the development of Mayaro virus diagnostics and in vaccine development and R&D.
Recombinant gene product from Mayaro virus, corresponding to amino acid 325-691 of the structural polyprotein. A glycine-serine linker and human IgG1 Fc-tag was fused to the C-terminus of this recombinant protein. We also offer alternative versions with mouse Fc-tag or His-tag to offer researchers maximum flexibility in their choice of reagent.
It has been reported that E2 protein may offer higher sensitivity in serological assays for alphaviruses when compared to E1 protein (Cho et al, 2008).
PRODUCT DETAILS – MAYARO VIRUS E2 ENVELOPE PROTEIN
- Recombinant Mayaro virus E2 Envelope protein (NCBI Accession Number: AJA37502.1).
- Includes amino acids 325-691 and a C-terminal human Fc-tag.
- Presented in Dulbecco’s phosphate buffered saline pH7.4, sterile filtered.
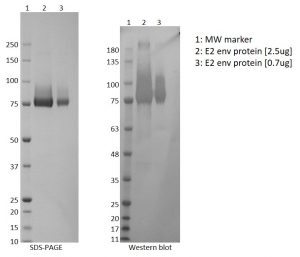
- Greater than 90% purity by SDS-PAGE.
BACKGROUND
Mayaro virus (MAYV) is a positive-sense single stranded RNA virus that belongs to the genus Alphavirus, a member of the Togaviridae family of viruses. It is a member of the Semliki Forest antigenic sero-complex, a serological group within the Alphavirus genus, and is closely related to Chikungunya virus (CHIKV) (Esposito, D.L.A).
MAYV is recognised as an emerging virus with the potential to cause a major epidemic in Central and South American countries. Currently, there is no licensed prophylactic vaccine or specific treatment for MAYV fever. Prevention of MAYV is through vector control measures to reduce transmission of the virus. Given the geographical distribution of MAYV and the similarity of the symptoms of Mayaro fever to infections caused by other arboviruses such Dengue fever, Chikungunya and Zika virus, it is considered important to be able to differentiate diagnostically between these arboviral diseases (CDC). Diagnosis of MAYV infection may be achieved by serological testing for MAYV specific IgM antibodies using enzyme immunoassays (EIA). However, cross reactivity with related viruses can reduce assay sensitivity and prevent accurate diagnosis (Figueiredo, ML).
REFERENCES
- Esposito DLA and Fonseca BALD (2017). Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Braz J Infect Dis.21(5):540-544
- Figueiredo ML and Figueiredo LT (2014). Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop.47(6):677-83