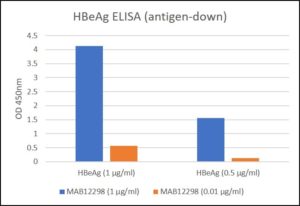
ELISA: Hepatitis B virus E antigen (HBeAg) coated at 0.5µg/ml and 1µg/ml at RT in DPBS for an hour. Plate then washed 1 X 300µl/well TBS + 0.1% Tween20, blocked 300µl/well DPBS+1% BSA 1h at room temperature. Detection antibody diluted to 1.0µg/ml and 0.01µgml in DPBS + 1% BSA + 0.05% T20. Added at 100ul/well, incubated shaken 2h room temperature. Plate washed 3 X 300µl/well TBS-T wash buffer. Biorad goat anti-mouse IgG-HRP (103005) secondary antibody diluted 1 in 10000 in DPBS/1%BSA/0.05%T20, added at 100µl/well, incubated shaken 1h room temperature. Plate washed 6X 300µl/well TBS-T wash buffer. Detection with KPL/Seracare SureBlue TMB substrate added at 100µl/well and the plate developed for 5 min. static on the bench. Reaction stopped with 100µl/well 1M HCL and the plate was read within 3.5 min. at 450nm.
Mouse Anti-Hepatitis B Virus E Antigen Antibody (M132)
$430.33 – $1,080.78 excl. VAT
MOUSE ANTI-HEPATITIS B VIRUS E ANTIGEN ANTIBODY (M132)
Mouse anti HBeAg antibody (M132) is specific for Hepatitis B virus E antigen. The Mouse Anti-Hepatitis B antibody is suitable for immunoassay research and development. Clone M132 (MAB12298) is suitable for use as a detection antibody with clone M133 (MAB12299) in ELISA assays.
PRODUCT DETAILS – MOUSE ANTI-HEPATITIS B VIRUS E ANTIGEN ANTIBODY (M132)
- Mouse anti-Hepatitis B virus E antigen monoclonal IgG2b antibody (clone M132).
- Greater than 90% purity by SDS-PAGE and buffered in 0.015M KPO4, 0.85% NaCl, pH 7.2.
BACKGROUND
Hepatitis B virus (HBV) is an enveloped double-stranded DNA virus, with a 3.2-kb covalently closed circular DNA, that belongs to the genus Orthohepadnaviruses of the Hepadnaviridae family of viruses. Currently, eight genotypes of HBV are recognised, designated A-H, with each genotype having a distinct geographical distribution. HBV is a retrovirus that replicates by reverse transcription of an RNA intermediate. Four genes are present on the virus genome; precore/core, polymerase (P), envelope (preS1/preS2/S) and X. The precore/core gene is responsible for expression of core protein as well as hepatitis B e antigen (HBeAg), a serological marker of HBV infection correlating with high viral load (Tong et al., 2005).
HBV chronically infects 300 million people worldwide, and increases their risk to develop hepatocellular carcinoma by a hundred fold. It is a bloodborne virus that is transmitted through contact with infected blood or bodily fluids. HBV infection may occur through various routes including the sharing of needles for injecting drugs, the use of inadequately sterilised medical equipment infected with HBV and the transfusion of unscreened blood and blood products. In endemic areas, perinatal transmission of HBV from mother to child is common.
HBV infection causes liver disease which can vary from acute, or chronic hepatitis to cirrhosis of the liver and potentially hepatocellular carcinoma. The incubation of HBV infection can vary from 1 – 6 months. During the period of acute infection, most individuals remain asymptomatic. However, some patients develop acute illness presenting with clinical symptoms that include jaundice, nausea, vomiting, abdominal pain and extreme fatigue. Acute liver failure may occur in 1% of patients, which can be fatal. HBV infected patients may also develop chronic lifelong disease, which can progress to cirrhosis or hepatocellular carcinoma in 20-30% of adult cases (WHO).
The asymptomatic nature of HBV infection, and the similarity of clinical symptoms to other types of hepatitis virus infection makes clinical diagnosis difficult. Therefore, laboratory diagnosis is undertaken using serological and molecular methods to detect HBsAg and specific IgM antibodies recognising core antigen HbcAg. The e-antigen is found in the blood only when there are viruses also present and is often used as a marker of ability to spread the virus to other people (infectivity). Measurement of e-antigen is used to monitor the effectiveness of HBV treatment, where successful treatment will usually eliminate HBeAg from the blood and lead to development of antibodies against e-antigen (anti-HBe). However, there are some types (strains) of HBV that do not make e-antigen, particularly in the Middle East and Asia, where testing for HBeAg is less useful.
REFERENCES
- Liang TJ.(2009). Hepatitis B: the virus and disease. Hepatology.May;49(5 Suppl): S13-21
- Seeger C, Mason WS. 2015. Molecular biology of hepatitis B virus infection. Virology.May;479-480:672-86.
- World health Organization: Factsheet, Hepatitis B