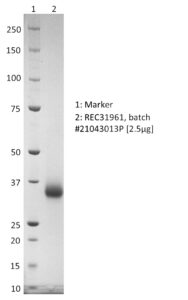
SDS-PAGE: Coomassie-stained SDS-PAGE showing purified SARS-CoV-2 RBD with K417T, E484K and N501Y mutations.
SARS-CoV-2 (B.1.1.28) Spike Glycoprotein (S1) RBD, His-Tag (HEK293)
$652.44 – $2,486.76 excl. VAT
BRAZILIAN (GAMMA) VARIANT
SARS-CoV-2 spike receptor binding domain (RBD) from Brazilian variant. Contains amino acid changes K417T, E484K, N501Y relative to Wuhan Hu-1. The protein was produced in HEK293 cells and purified from culture supernatant. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
SARS-COV-2 (B.1.1.28) SPIKE GLYCOPROTEIN (S1) RBD, HIS-TAG (HEK293)
SARS-CoV-2 (B.1.1.28) RBD mutant from Brazilian variant. Contains amino acid changes K417T, E484K, N501Y relative to Wuhan Hu-1. The protein was produced in HEK293 cells and purified from culture supernatant. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
PRODUCT DETAILS – SARS-COV-2 (B.1.1.28) SPIKE GLYCOPROTEIN (S1) RBD, HIS-TAG (HEK293)
- SARS-CoV-2 (B.1.1.28) spike RBD.
- SARS-CoV-2 (B.1.1.28) RBD contains K417T, E484K, N501Y mutations relative to Wuhan Hu-1.
- Expressed in HEK293 and purified by affinity chromatography.
- Presented in DPBS at
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes coronavirus disease 2019 (COVID-19). The sequence WIV04/2019, belonging to the GISAID S clade / PANGOLIN A lineage / Nextstrain 19B clade, is believed to be the original sequence infecting humans (Zhukova et al., 2020). However, there are many thousands of variants of SARS-CoV-2 (Koyama et al., 2020) and subtypes of the virus can be placed into much larger groupings such as lineages or clades.
In Brazil, a variant of SARS-CoV-2 known as P.1 (20J/501Y.V3, Variant of Concern 202101/02) emerged that was first was identified in four travelers from Brazil, who were tested during routine screening at Haneda airport outside Tokyo, Japan. The P.1 variant contains 17 non-synonymous mutations [L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, and V1176F] in S protein, [S1188L, K1795Q, and E5665D] in ORF1ab, [E92K] in ORF8, and [P80K] in N protein; 1 deletion: [SGF 3675-3677del] in ORF1ab; and 4 synonymous mutations. P.1 is the SARS-CoV-2 variant that accumulates the highest number of mutations in the spike protein (12 mutations) (Gómez et al., 2021). The P.1 variant is found mainly in outbreaks in and around Manaus, the capital of the Brazilian state of Amazonas. A second Brazilian P.2 lineage (B.1.1.28.2 or VUI-202101/01) originated in Rio de Janeiro. This variant only carries the E484K mutation and does not have N501Y or K417T. The P.2 variant occurs throughout Brazil.
One of the most worrying mutations in terms of immune evasion is the E484K, which is shared by the P.1 and the B.1.351 variants. The effect of this mutation has been shown to reduce neutralization ability of sera from convalescent or vaccinated patients. However, sera with high anti-S IgG titers were still able to neutralize the virus with the mutation, suggesting that it is important to induce the highest possible levels of specific antibodies through vaccination to improve protection against emerging variants of SARS-CoV-2 (Jangra et al., 2021). There is evidence to suggest that some of the mutations in the P.1 variant may affect its transmissibility and antigenic profile, which may affect the ability of antibodies generated through a previous natural infection or through vaccination to recognize and neutralize the virus. This has important implications for transmission, reinfection rates, and evasion of antibody-mediated immunity.
REFERENCES
- CDC. Science Brief: Emerging SARS-CoV-2 Variants. Jan. 28, 2021.
- Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines (Basel). 2021 Mar 11;9(3):243.
- Jangra, S.; Ye, C.; Rathnasinghe, R.; Stadlbauer, D.; PVI Study Group; Krammer, F.; Simon, V.; Martinez-Sobrido, L.; García-Sastre, A.; Schotsaert, M. The E484K Mutation in the SARS-CoV-2 Spike Protein Reduces but Does Not Abolish Neutralizing Activity of Human Convalescent and Post-Vaccination Sera. medRxiv 2021.
- Koyama T, Platt D, Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ. 2020 Jul 1;98(7):495-504.
- Zhukova A, Blassel L, Lemoine F, Morel M, Voznica J, Gascuel O. Origin, evolution and global spread of SARS-CoV-2. C R Biol. 2020 Nov 24. doi: 10.5802/crbiol.29.