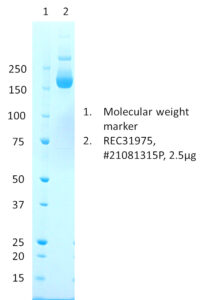
SDS-PAGE: Coomassie-stained SDS-PAGE showing purified SARS-CoV-2 (B.1.617.2; AY.1, AY.2, AY.3) spike protein.
SARS-CoV-2 (B.1.617.2; AY.1, AY.2, AY.3) Stabilized Spike Glycoprotein (Trimeric), His-Strep-Tag (HEK293)
$989.55 – $3,763.85 excl. VAT
INDIAN (DELTA) VARIANT
SARS-CoV-2 (B.1.617.2; AY.1, AY.2, AY.3) stabilized Spike from the Indian (Delta) variant. Contains T19R, T95I, G142D, E156G, del157-158, W258L, N417K, L452R, T478K, D614G, P681R, D950N mutations. The protein was produced in HEK293 cells and purified from culture supernatant. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
SARS-COV-2 (B.1.617.2; AY.1, AY.2, AY.3) STABILIZED SPIKE GLYCOPROTEIN (TRIMERIC), HIS-STREP-TAG (HEK293)
SARS-CoV-2 (B.1.617.2 AY.1 AY.2 AY.3) stabilized spike from the Indian (Delta) variant. Contains T19R, T95I, G142D, E156G, del157-158, W258L, N417K, L452R, T478K, D614G, P681R, D950N mutations. The protein was produced in HEK293 cells and purified from culture supernatant. SARS-CoV-2, previously known as the 2019 Novel Coronavirus (2019-nCoV), causes the pandemic COVID-19 disease.
PRODUCT DETAILS – SARS-COV-2 (B.1.617.2; AY.1, AY.2, AY.3) STABILIZED SPIKE GLYCOPROTEIN (TRIMERIC), HIS-STREP-TAG (HEK293)
- SARS-CoV-2 (B.1.617.2 AY.1 AY.2 AY.3) stabilized spike protein (Accession: 6XKL_A)
- This spike protein contains T19R, T95I, G142D, E156G, del157-158, W258L, N417K, L452R, T478K, D614G, P681R, D950N mutations.
- The protein is stabilized as a pre-fusion trimer due to six amino acid changes in the S2 portion of Spike (HexaPro). The furin cleavage site has been mutated to GSAS.
- Expressed in HEK293 and purified by affinity chromatography.
- Presented in DPBS at
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes coronavirus disease 2019 (COVID-19). The sequence WIV04/2019, belonging to the GISAID S clade / PANGOLIN A lineage / Nextstrain 19B clade, is believed to be the original sequence infecting humans (Zhukova et al., 2020). However, there are many thousands of variants of SARS-CoV-2 (Koyama et al., 2020) and subtypes of the virus can be placed into much larger groupings such as lineages or clades. WHO have designated variants of concern as Alpha (United Kingdom), Beta (South Africa), Gamma (Brazil) and Delta (India), depending on location of earliest documented samples.
B.1.617, also known as the “double mutant” variant, was first detected in India in October 2020. Since then, three sub lineages of this variant have been detected, namely B.1.617.1, B.1.617.2 and B.1.617.3. The B.1.617.2 is extremely transmissible and is the fourth strain of the SARS-CoV-2 virus to have undergone a mutation toward a more virulent and transmissible form. The B.1.617 lineage possesses 13 to 17 mutations, three of which are in the virus’ spike protein. B.1.617.2 has mutations in the gene encoding the SARS-CoV-2 spike protein causing the substitutions T478K, P681R and L452R, which are known to affect transmissibility of the virus as well as whether it can be neutralized by antibodies for previously circulating SARS-CoV-2 variants. The L452R mutation confers stronger affinity of the spike protein for the ACE2 receptor and decreased recognition capability of the immune system. Public Health England (PHE) have assessed transmissibility as equivalent to B.1.1.7 (Alpha variant), first identified in the UK (Kent variant). The variant is thought to be partly responsible for India’s second wave of the pandemic beginning in February 2021. It later contributed to a third wave in the United Kingdom and South Africa (WHO; CDC). The Delta variant causes more infections and spreads faster than earlier forms of the virus that causes COVID-19 and may cause more severe illness than previous strains in unvaccinated people. It shows increased transmissibility, potential reduction in neutralization by some EUA monoclonal antibody treatments and potential reduction in neutralization by post-vaccination sera (CDC). The Delta variant also contains several different clades or sub-lineages (based on mutations), corresponding to the Pango lineages AY.1 (Delta Plus), AY.2 and AY.3.
REFERENCES
- CDC. SARS-CoV-2 Variant Classifications and Definitions.
- Koyama T, Platt D, Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ. 2020 Jul 1;98(7):495-504.
- Zhukova A, Blassel L, Lemoine F, Morel M, Voznica J, Gascuel O. Origin, evolution and global spread of SARS-CoV-2. C R Biol. 2020 Nov 24. doi: 10.5802/crbiol.29.
- WHO. Tracking SARS-CoV-2 variants.