BacTrace® Antigens and Antibodies
BacTrace® antigens and antibodies are ideal for developing high performance assays for the detection of bacterial pathogens.
Background
LGC Clinical Diagnostics’ KPL portfolio includes a range of over 800 reagents and kits for immunoassays, immunohistochemistry, infectivity assays and other applications.
BacTrace comprises KPL’s range of native bacterial antigens and matching primary antibodies. Produced in an ISO13485-certified facility, BacTrace bacterial antigens are grown as whole cells in native culture. Cells are then heat-inactivated and validated in infectivity assays, making them safe-to-use, while presenting a broad range of surface epitopes.
BacTrace antibodies are generated by inoculating goats with BacTrace bacterial antigens. The resulting polyclonal sera then undergoes a proprietary affinity purification protocol that preserves target antigen affinity while yielding fractions that are highly specific to the pathogen in question. Combined with downstream cross-reactivity testing, BacTrace antibodies benefit from maximal signal-to-noise ratio, while ensuring defined specificity and lot-to-lot consistency.
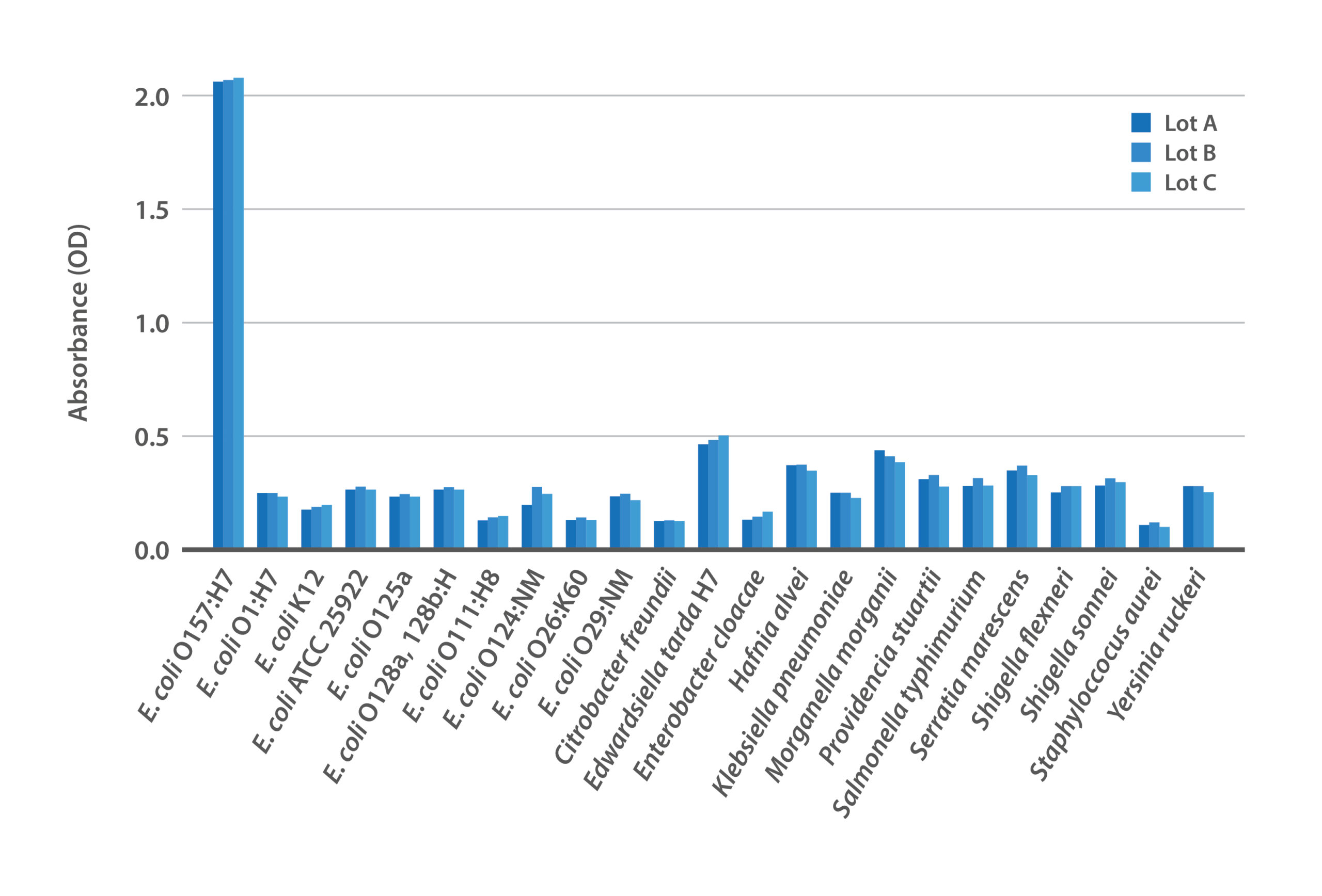
Figure 1: Lot-to-lot consistency of BacTrace® anti-E. coli O157:H7 polyclonal antibody against panel of bacteria.
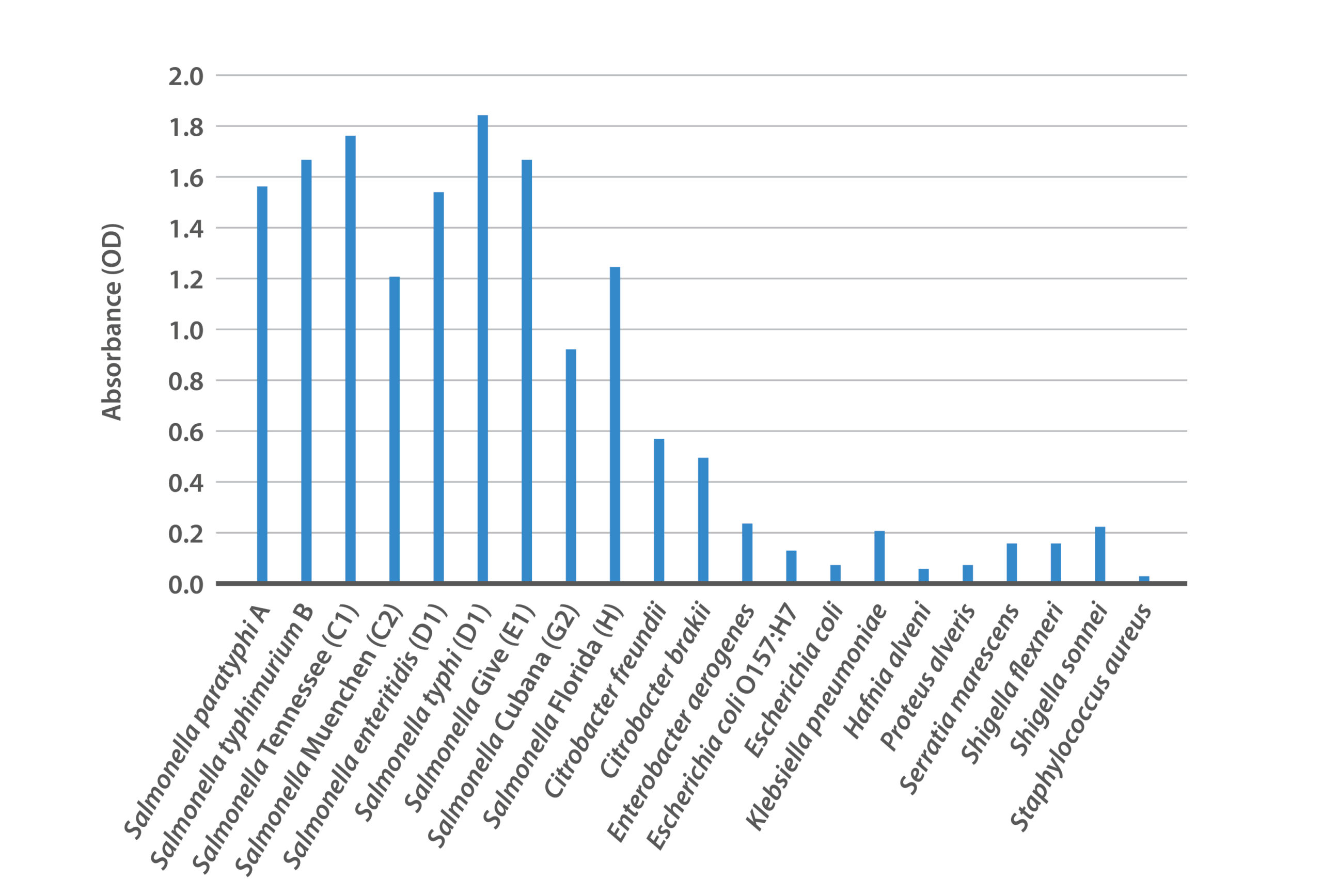
Figure 2: Reactivity of BacTrace® anti-Salmonella polyclonal antibody against panel of Salmonella serotypes and other bacteria.
BacTrace® Products
Our BacTrace® range includes antigens and antibodies for a number of notable food-borne pathogens, as well as other bacterial targets, including:
About KPL
KPL was acquired by SeraCare Life Sciences in 2013, which was in turn acquired by LGC in 2018 and is now part of LGC Clinical Diagnostics, alongside The Native Antigen Company.
KPL is a pioneer in the development of large-scale affinity-purified polyclonal antibodies for use in a range of applications. Using proprietary technologies that have been continually refined since the company’s founding in 1979, KPL stands among the industry’s most trusted and reliable sources of high-performance antibodies.
