Yellow fever is a haemorrhagic viral disease, transmitted by infected mosquitos in Africa and South America. While an effective vaccine is available, inadequate vaccination of endemic areas has made diagnostics a necessity to controlling ongoing outbreaks. However, accurate diagnostics of yellow fever are currently limited by assays that are either unreliable or too impractical for effective outbreak response. Here, we explore the ecology of the Yellow Fever virus, the need for improved diagnostics and the development of effective alternatives to existing assays.
Yellow Fever Virus
The Yellow Fever Virus (YFV) is a 40-50nm, enveloped RNA virus of the Flaviviridae family, which includes Dengue virus, West Nile virus, Zika virus, Japanese Encephalitis virus and Tick-Borne Encephalitis virus.
YFV causes yellow fever – an acute haemorrhagic disease, so called because of the jaundice associated with liver dysfunction. Once an individual is infected with the disease, management is symptomatic with no effective means of treating the infection. Symptoms include jaundice, fever, headache, muscle pain, vomiting, nausea and fatigue and 15% of those who contract YFV develop severe symptoms, of which up to 50% die within 10 days1. The true incidence of yellow fever is unknown, due to insufficient reporting, surveillance and limited access to effective diagnostics.
Transmission and Ecology
YFV is predominantly transmitted by Aedes aegypti mosquitos, but is carried by a wide range of species. YFV is first acquired by female mosquitos when feeding on the blood of infected humans or other primates, which then reach the mosquito’s stomach. Sufficient viral concentrations then permit the virus to infect the mosquito’s epithelial cells and replicate. From here, YFV moves to the mosquito’s circulatory system and salivary glands, so that when the mosquito next feeds, it injects its saliva into the wound and infects the host.
Yellow fever is now endemic in 44 countries across the tropics and subtropics of Africa and South America where viral circulation is propagated by the natural presence of mosquito and primate vectors2. Various mosquitoes feed on infected primates in the forests of Africa and South America and transmit YFV to humans that enter these forests – most commonly from sylvatic mosquitos that occasionally circulate in village populations at the fringes of woodlands.
The greatest risk of epidemic occurs when infected humans then return to urban areas (often for medical treatment) and are bitten by the Aedes aegypti mosquito, which are then able to rapidly propagate the virus in urban environments.
Three epidemiologically distinct infectious cycles for the yellow fever virus are known to occur:
1. In the sylvatic (forest) cycle, non-aegypti species serve as vectors to transmit YFV to mainly non-human primates, resulting in asymptomatic infection.
2. In the urban cycle, only the Aedes aegypti mosquito is involved, as it is well adapted to the urban areas of Africa – continually transmitting YFV and infecting human hosts.
3. In Africa, the savannah cycle acts as an intermediate between the urban and sylvatic cycles where a variety of Aedes species directly transmit YFV between humans and other primates.
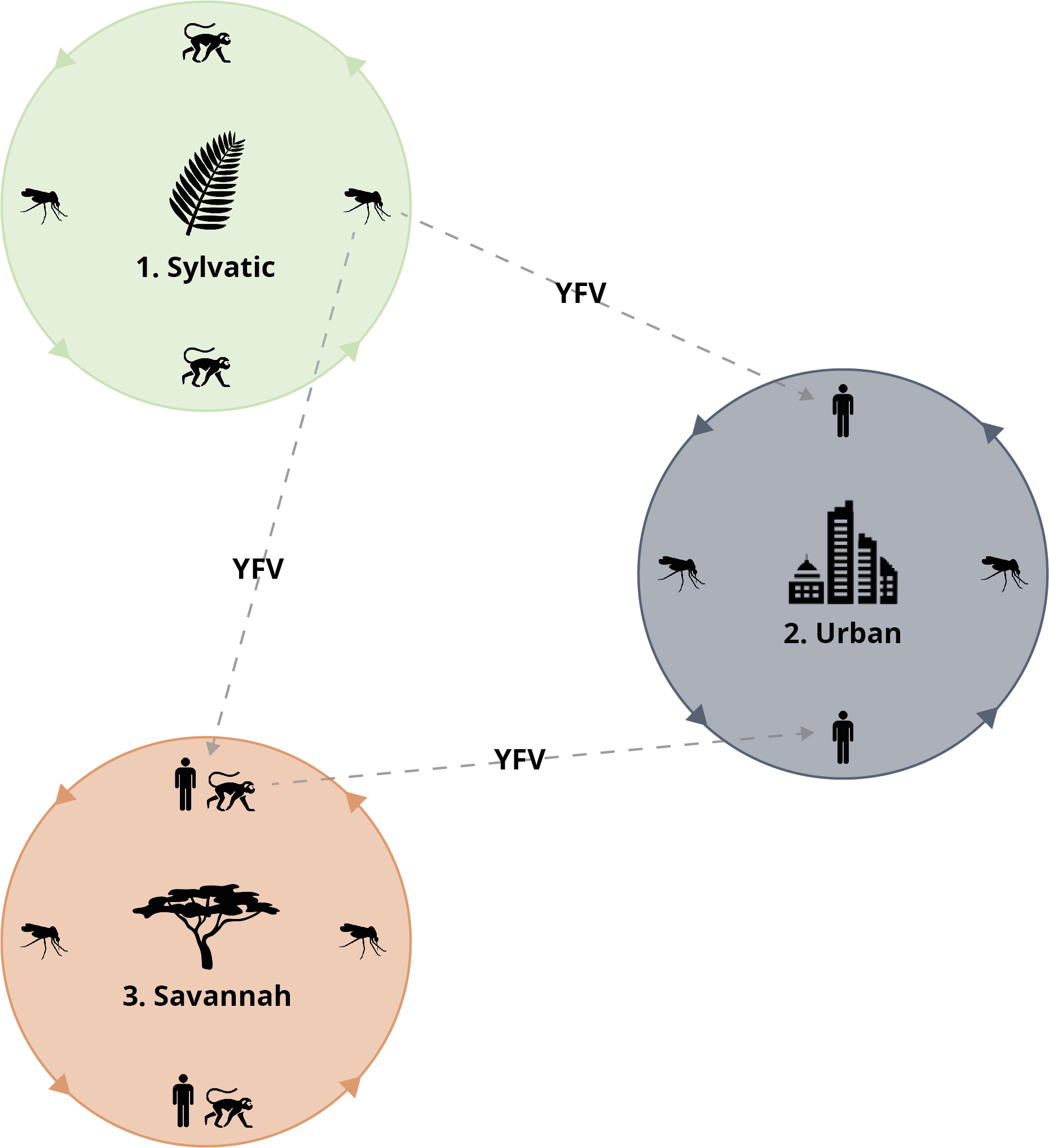
The Yellow Fever virus transmission cycle. Credit: WHO.
In 2015-2016, urban outbreaks of yellow fever were reported in the Democratic Republic of Congo and Angola and there has been an ongoing sylvatic outbreak in Brazil since 20163. Of particular concern is the risk of an urban transmission cycle developing in Brazil and spreading to countries with viable vectors and susceptible populations – a very real threat, considering the expansive distribution of the A. aegypti mosquito.
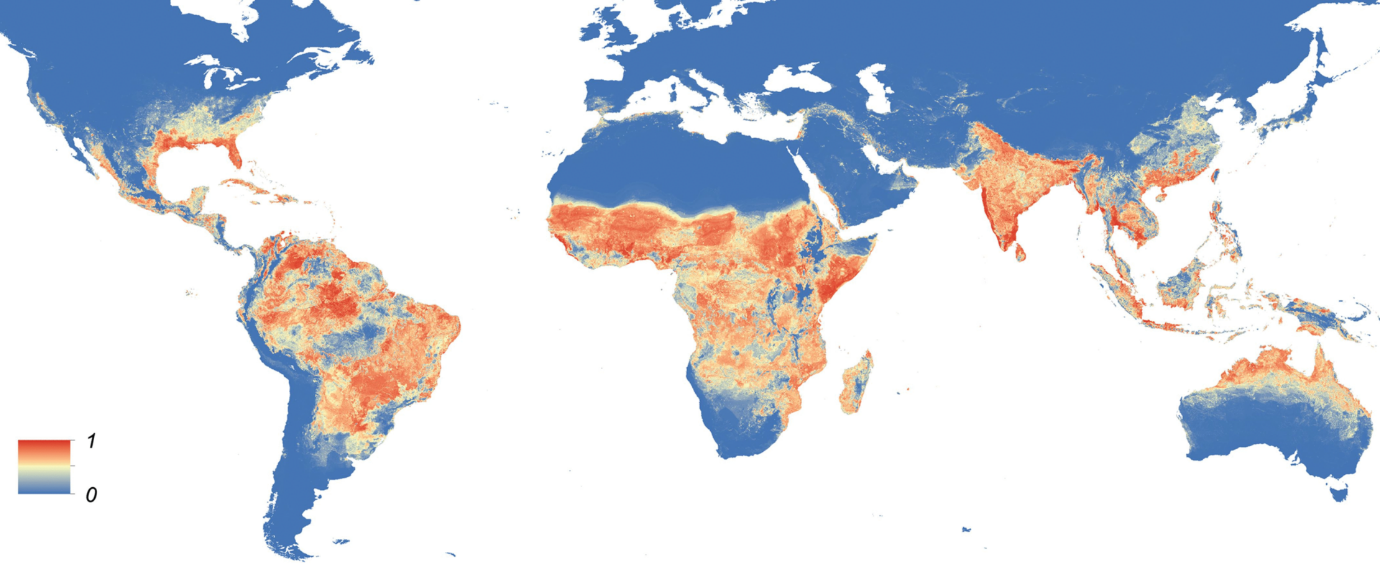
World map, predicting the global distribution of Aedes aegypti. Credit: Kraemer et al.4.
A Yellow Fever Vaccine
An effective yellow fever vaccine has been available since the 1930s and has proven to be safe and affordable1. Since its introduction, the vaccine has been successfully used to immunize populations where YFV is endemic, but coverage is sparse and lack of immunity continues to contribute to sporadic outbreaks5. Furthermore, low stockpiles have forced more frugal immunisation strategies and the WHO estimates that 900 million people remain at risk in endemic areas, of which the majority live in African countries6. Therefore, despite the availability of an effective vaccine, yellow fever remains a public health issue in both endemic and naive areas3.
The EYE Strategy
In 2017, the Eliminate Yellow fever Epidemics (EYE) strategy was launched as an international collaboration to protect at-risk populations, contain outbreaks and ensure affordable vaccine access7. As part of the strategy, EYE has called for priority research into better diagnostic capabilities for early detection and rapid response, to mitigate further transmission8.
In particular, the recent epidemic in Angola has highlighted the effect of substantially limited surveillance and laboratory capacities contributing to accelerated and unnecessary spread of yellow fever. Due to delays in detection of cases, the outbreak reached a magnitude that required disproportionately greater containment measures and allowed international spread by unimmunised travellers.
As efforts continue to improve immunity levels in endemic populations, outbreaks will likely continue to occur. Therefore, strengthened surveillance programs should be in place for rapid response and containment efforts to outbreaks8. For surveillance programs to function effectively, better diagnostic capacities are needed to provide more information, necessary for improved assessment of evolving risk, as well as the impact of preventive and control measures. Robust diagnostics also facilitate better understanding of yellow fever’s geographical risk to optimise allocation of limited resources.
Challenges to Yellow Fever Diagnostics
Accurate clinical diagnosis of yellow fever is inherently challenging, as early symptoms of infection are easily confused with other haemorrhagic viral diseases, such as Dengue, Zika, Ebola, Lassa and Marburg. All of these diseases need to be considered in differential diagnoses and laboratory confirmation is crucial. However, there is limited availability of commercial assays, relevant data and well-defined validation panels, which collectively continue to hamper the control of this disease.
A notorious issue in developing effective assays is cross-reactivity between the flaviviruses, owing to their similar antigenic makeup. Assays that are unable to reliably distinguish between flavivirus antigens result in false positives and misdiagnoses of patients, emphasising the need for effective differential diagnosis in areas where multiple flaviviruses such as YFV, Dengue and Zika co-circulate. Surprisingly, few diagnostic assays have been extensively validated with clinical samples from natural yellow fever infections against different co-circulating viruses.
There are a range of different assay formats that can be used to diagnose a yellow fever infection:
Plaque Reduction Neutralisation (PRNT) assays or Virus Neutralisation Tests (VNT) are regarded as the gold standard for differential diagnosis of flaviviruses, owing to their high specificity. However, PRNT assays require level BSL-3 biocontainment facilities, experienced staff and standardised control procedures to produce accurate and reliable results. This limits PRNT assays to reference laboratories, creating severe bottlenecks in outbreak scenarios that are unable to cope with the volume of necessary testing. This is further compounded by the time taken to carry out PRNT assays, which can take up to one week to produce results. As the asymptomatic incubation period of YFV is relatively long, such timescales are not suited to the rapid decision-making needed during outbreak responses to prevent further infections.
Serological assays for yellow fever infection detect either IgM antibodies specific to YFV or a 4x elevated increase in anti-YFV IgG. Yellow fever serological diagnosis is complicated by cross-reactivity with other flavivirus members. In particular, prior Dengue Virus immunity frequently generates non-specific false positives in current serological methods – a result of a phenomenon known as original antigenic sin. Thus, serological tests are unable to reliably distinguish cross-reactive flavivirus antibodies between natural and vaccine-acquired immunity.
Because of the practical limitations in using PRNT or serological assays, more rapid and reliable methods are needed for the effective diagnosis of YFV in outbreak scenarios.
Enzyme-linked immunosorbent assays, or ELISAs are a widely-available plate-based assay that can be used to detect the presence of antigens with antibodies. Antibodies are enzyme-linked to produce a detectable signal upon binding with an antigen, signifying the presence of the virus. As antibodies have a very strong affinity to their corresponding antigens, ELISAs show very high specificity, allowing quantification at the microgram or even nanogram level. ELISAs are also rapid – typically producing results within 4-5 hours and are robust enough for use in the most basic of laboratory settings.
Another growing technology in disease diagnostics are lateral flow devices, or LFDs. These devices are very simple, using a paper base, across which the sample (analyte) flows and binds to corresponding antibodies to produce a colour change at one of multiple locations. A well-known example of an LFD is the home pregnancy test. A key advantage of LFDs over other assays are their ability to be used in the field – ideal in low-resource settings and for point-of-care treatment in outbreak scenarios. LFDs are also very simple to use, requiring little-to-no training and yield results in a matter of minutes.
Yet, while both ELISAs and LFDs are strong candidates for YFV diagnostics, they are hindered by their inability to reliably distinguish members of the flaviviridae family, resulting from cross-reactivity between flavivirus antigens and non-specific antibodies.
NS1 Protein ELISAs
The Yellow Fever virus encodes 3 structural and 7 non-structural proteins. Of particular interest is non-structural protein 1 (NS1) – a glycoprotein found on the membrane of infected cells as a monomer, and in extracellular secretions and blood serum as a hexamer (sNS1). As sNS1 can be readily detected in the blood serum of patients (unlike other viral components), it is gaining traction as a practical candidate for flavivirus immunoassays.
However, NS1 proteins share strong sequence similarity in the flavivirus genus, allowing non-specific antibodies to readily reacting with a range of NS1 proteins and produce false-positive results in immunoassays9. It is therefore crucial to use highly specific monoclonal antibodies for the accurate detection of YFV and reliably distinguish it from other, related viruses.
The presence of secreted NS1 proteins in the patient sera of those infected with West Nile virus and Dengue virus have proven to be effective markers of early infection, owing to the use of highly specific anti-NS1 monoclonal antibodies. A more recent paper by Ricciardi-Jorge et al. reported 80% sensitivity, 100% specificity and 100% positive predictive value using a monoclonal antibody-specific NS1 ELISA for YFV10. In light of these results, commercially-available NS1 assays are prime contenders to meet current diagnostic challenges in the fight against yellow fever.
Reagents from The Native Antigen Company
The Native Antigen Company is supporting ongoing research into Yellow Fever Virus, with an extended range of antigens, antibodies and YFV donor sera for use in diagnostic tests and vaccine research.
Using our proprietary mammalian cell expression system, we have generated highly pure NS1 protein in its hexameric conformation, with native glycosylation and folding. Our yellow fever NS1 proteins are ideal for use as controls and standards in ELISA, LFD and related tests for measuring patient NS1 in early infection – an excellent target for developing serological assays.
Using our recombinant YFV NS1 proteins, we have also raised a panel of antibodies to NS1, all of which are highly specific and show no cross-reactivity with other flaviviral NS1 proteins, including Dengue, Zika, West Nile and Japanese Encephalitis viral proteins. These antibodies are perfect for the development of immunoassays to measure the level of YFV NS1 proteins.
We are also pleased to announce the release of a unique panel of YFV donor sera. Sera has been collected from a panel of donors who have been immunised with the Yellow Fever vaccine, Stamaril, with samples available from each donor prior to vaccination, and at 2-week and 6-week time points, to allow for assessment of IgM and IgM responses. Our YFV sera is appropriate for use in diagnostic immunoassay controls, for both YFV assays and to investigate assay cross-reactivity with other flaviviruses.
References
- http://www.who.int/en/news-room/fact-sheets/detail/yellow-fever
- https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/yellow_fever/en/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6043483/
- https://elifesciences.org/articles/08347
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027470/
- http://www.who.int/csr/disease/yellowfev/yellow-fever-initiative/en/
- http://www.who.int/csr/disease/yellowfev/meeting-demand-for-vaccines/en/
- http://apps.who.int/iris/bitstream/handle/10665/272408/9789241513661-eng.pdf?ua=1
- https://www.ncbi.nlm.nih.gov/pubmed/27473856
- https://www.nature.com/articles/s41598-017-16231-6

